Abstract
Background
Ribosomal protein L38 (RPL38) was found upregulated in osteoarthritic peripheral blood mononuclear cells, however, its role in progression of osteoarthritis has not been characterized.
Methods
The protein levels of RPL38 and SOCS2 in cartilage tissues from OA patients and controls were detected with Western blotting. IL-1β was used to stimulate primary chondrocytes to establish an OA cell model, and RPL38 siRNA (si-RPL38) was transfected into chondrocytes to investigate the effect of RPL38 knockdown on cell viability, apoptosis, inflammatory factor secretion and extracellular matrix degradation. Then, the mechanism that RPL38 regulate the SOCS2 expression and SOCS2-induced chondrocyte dysfunction was explored. The methyltransferase-like 3 (METTL3)-mediated m6A modification of SOCS2 mRNA was confirmed, and the interaction of RPL38 and METTL3 was verified. Moreover, the effects of SOCS2 overexpression on IL-1β-induced chondrocyte dysfunction and SOCS2 knockdown on the restoration of chondrocyte function by siRPL38 were investigated. Finally, RPL38 was knocked down in vivo and its role in OA progression was validated.
Results
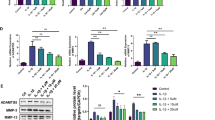
RPL38 was upregulated and SOCS2 was downregulated in OA cartilages. RPL38 knockdown or SOCS2 overexpression either attenuated IL-1β-induced chondrocyte apoptosis, inflammatory cytokine secretion, and ECM degradation. RPL38 directly interacted with METTL3 and it inhibited SOCS2 expression through METTL3-mediated m6A modification. SOCS2 knockdown activated the JAK2/STAT3 proinflammatory pathway and reversed the effects of RPL38 knockdown on IL-1β-induced chondrocyte apoptosis, inflammation and ECM degradation. RPL38 knockdown alleviated cartilage tissue damage and ECM degradation in OA mice.
Conclusion
RPL38 knockdown inhibited osteoarthritic chondrocyte dysfunction and alleviated OA progression through promoting METTL3-m6A-mediated SOCS2 expression.






Similar content being viewed by others
Availability of materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
References
Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthr Cartil. 2018;26(3):319–25.
Singh P, Marcu KB, Goldring MB, et al. Phenotypic instability of chondrocytes in osteoarthritis: on a path to hypertrophy. Ann NY Acad Sci. 2019;1442(1):17–34.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59.
Li B, Guan G, Mei L, et al. Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint. J Cell Mol Med. 2021;25(11):4902–11.
Shahid M, Manchi G, Slunsky P, et al. A systemic review of existing serological possibilities to diagnose canine osteoarthritis with a particular focus on extracellular matrix proteoglycans and protein. Pol J Vet Sci. 2017;20(1):189–201.
Hoshi H, Akagi R, Yamaguchi S, et al. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017;368(2):379–87.
Sahin F, Qiu W, Wilentz RE, et al. RPL38, FOSL1, and UPP1 are predominantly expressed in the pancreatic ductal epithelium. Pancreas. 2005;30(2):158–67.
Zheng B, Peng J, Mollayup A, et al. Construction of a prognostic prediction system for pancreatic ductal adenocarcinoma to investigate the key prognostic genes. Mol Med Rep. 2018;17(1):216–24.
Ji H, Zhang X. RPL38 regulates the proliferation and apoptosis of gastric cancer via miR-374b-5p/VEGF signal pathway. Onco Targets Ther. 2020;13:6131–41.
Shi T, Shen X, Gao G. Gene expression profiles of peripheral blood monocytes in osteoarthritis and analysis of differentially expressed genes. Biomed Res Int. 2019;2019:4291689.
Rico-Bautista E, Flores-Morales A, Fernández-Pérez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev. 2006;17(6):431–9.
Val CH, de Oliveira MC, Lacerda DR, et al. SOCS2 modulates adipose tissue inflammation and expansion in mice. J Nutr Biochem. 2020;76: 108304.
Toscano ECB, Sousa L, Lima GK, et al. Neuroinflammation is associated with reduced SOCS2 and SOCS3 expression during intracranial HSV-1 infection. Neurosci Lett. 2020;736: 135295.
de Andrés MC, Imagawa K, Hashimoto K, et al. Suppressors of cytokine signalling (SOCS) are reduced in osteoarthritis. Biochem Biophys Res Commun. 2011;407(1):54–9.
Wang BW, Jiang Y, Yao ZL, et al. Aucubin protects chondrocytes against IL-1β-induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des Dev Ther. 2019;13:3529–38.
Wang X, Fan J, Ding X, et al. Tanshinone I inhibits IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in chondrocytes CHON-001 cells and attenuates murine osteoarthritis. Drug Des Dev Ther. 2019;13:3559–68.
Xu J, Chen Q, Tian K, et al. m6A methyltransferase METTL3 maintains colon cancer tumorigenicity by suppressing SOCS2 to promote cell proliferation. Oncol Rep. 2020;44(3):973–86.
Malemud CJ. Negative regulators of JAK/STAT signaling in rheumatoid arthritis and osteoarthritis. Int J Mol Sci. 2017;18(3):484.
Hada S, Kaneko H, Sadatsuki R, et al. The degeneration and destruction of femoral articular cartilage shows a greater degree of deterioration than that of the tibial and patellar articular cartilage in early stage knee osteoarthritis: a cross-sectional study. Osteoarthr Cartil. 2014;22(10):1583–9.
Zhang J, Cheng F, Rong G, et al. Circular RNA hsa_circ_0005567 overexpression promotes M2 type macrophage polarization through miR-492/SOCS2 axis to inhibit osteoarthritis progression. Bioengineered. 2021;12(1):8920–30.
Chen M, Wei L, Law CT, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67(6):2254–70.
Lim H, Kim HP. Matrix metalloproteinase-13 expression in IL-1β-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch Pharm Res. 2011;34(1):109–17.
Letellier E, Haan S. SOCS2: physiological and pathological functions. Front Biosci (Elite Ed). 2016;8:189–204.
Bao NN, Kong DY, Zhu D, et al. Influence of overexpression of SOCS2 on cells of DN rat. Asian Pac J Trop Med. 2015;8(7):583–9.
Funding
No applicable.
Author information
Authors and Affiliations
Contributions
HH designed the experiments. LS, HH, PS, ZL and LJ performed the experimental work. SL and JZ performed statistical analysis and prepared the figures for the manuscript. HH wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This study was approved by the Ethnic Committee of Shaanxi Provincial People's Hospital, and all patients had read and signed the informed consent. All animal care and experimental procedures were approved by the Ethics Committee of Shaanxi Provincial People's Hospital (SXSPPH-2020-022).
Additional information
Responsible Editor: Jason J. McDougall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, L., Hu, H., Sun, P. et al. RPL38 knockdown inhibits the inflammation and apoptosis in chondrocytes through regulating METTL3-mediated SOCS2 m6A modification in osteoarthritis. Inflamm. Res. 71, 977–989 (2022). https://doi.org/10.1007/s00011-022-01579-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01579-x