Abstract
Objective
The transient receptor potential vanilloid subtype 1 (TRPV1) channel is considered to play an important regulatory role in the process of pain. The purpose of this study is to observe the change characteristics of TRPV1 channel in MSU-induced gouty arthritis and to find a new target for clinical treatment of gout pain.
Methods
Acute gouty arthritis was induced by injection of monosodium urate (MSU) crystals into the ankle joint of mice. The swelling degree was evaluated by measuring the circumference of the ankle joint. Mechanical hyperalgesia was conducted using the electronic von Frey. Calcium fluorescence and TRPV1 current were recorded by applying laser scanning confocal microscope and patch clamp in dorsal root ganglion (DRG) neurons, respectively.
Results
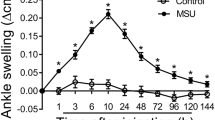
MSU treatment resulted in significant inflammatory response and mechanical hyperalgesia. The peak swelling degree appeared at 12 h, and the minimum pain threshold appeared at 8 h after MSU treatment. The fluorescence intensity of capsaicin-induced calcium response and TRPV1 current were increased in DRG cells from MSU-treated mice. The number of cells that increased calcium response after MSU treatment was mainly distributed in small-diameter DRG cells. However, the action potential was not significantly changed in small-diameter DRG cells after MSU treatment.
Conclusions
These findings identified an important role of TRPV1 in mediating mechanical hyperalgesia in MSU-induced gouty arthritis and further suggest that TRPV1 can be regarded as a potential new target for the clinical treatment of gouty arthritis.







Similar content being viewed by others
References
Richette P, Bardin T. Gout. Lancet. 2010;375(9711):318–28.
Tatlock S, Rudell K, Panter C, Arbuckle R, Harrold LR, Taylor WJ, et al. What outcomes are important for gout patients? In-depth qualitative research into the gout patient experience to determine optimal endpoints for evaluating therapeutic interventions. Patient. 2017;10(1):65–79.
Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364(5): 443–52.
Cronstein BN, Sunkureddi P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. J Clin Rheumatol. 2013;19(1):19–29.
Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6(1):30–8.
Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388(10055):2039–52.
Qaseem A, Harris RP, Forciea MA, Denberg TD, Barry MJ, Boyd C, et al. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(1):58–68.
Schlesinger N, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat gouty arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther. 2011;13(2):R53.
De Logu F, Geppetti P. Ion channel pharmacology for pain modulation. Handb Exp Pharmacol. 2019;260:161–86.
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13.
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24.
Zhang BY, Zhang YL, Sun Q, Zhang PA, Wang XX, Xu GY, et al. Alpha-lipoic acid downregulates TRPV1 receptor via NF-кB and attenuates neuropathic pain in rats with diabetes. CNS Neurosci Ther. 2020;26(7):762–72.
Yu L, Yang F, Luo H, Liu F, Han J, Xing G, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4(1):61.
Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29(1):355–84.
Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11(5):555–64.
Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, et al. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKC epsilon: a novel pathway for heat hyperalgesia. J Neurosci. 2007;27(44):12067–77.
Han P, Korepanova AV, Vos MH, Moreland RB, Chiu ML, Faltynek CR. Quantification of TRPV1 protein levels in rat tissues to understand its physiological roles. J Mol Neurosci. 2013;50(1):23–32.
Chakrabarti S, Pattison LA, Singhal K, Hockley JRF, Callejo G, Smith ESJ. Acute inflammation sensitizes knee-innervating sensory neurons and decreases mouse digging behavior in a TRPV1-dependent manner. Neuropharmacology. 2018;143:49–62.
Chen Y, Willcockson HH, Valtschanoff JG. Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthr Cartil. 2009;17(2):244–51.
Szabo A, Helyes Z, Sandor K, Bite A, Pinter E, Nemeth J, et al. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther. 2005;314(1):111–9.
Ishikura T, Suzuki H, Shoguchi K, Koreeda Y, Aritomi T, Matsuura T, et al. Possible involvement of TRPV1 and TRPV4 in nociceptive stimulation- induced nocifensive behavior and neuroendocrine response in mice. Brain Res Bull. 2015;118:7–16.
Watanabe M, Ueda T, Shibata Y, Kumamoto N, Ugawa S. The role of TRPV1 channels in carrageenan-induced mechanical hyperalgesia in mice. NeuroReport. 2015;26(3):173–8.
Yin C, Liu B, Wang P, Li X, Li Y, Zheng X, et al. Eucalyptol alleviates inflammation and pain responses in a mouse model of gout arthritis. Br J Pharmacol. 2020;177(9):2042–57.
Hoffmeister C, Silva MA, Rossato MF, Trevisan G, Oliveira SM, Guerra GP, et al. Participation of the TRPV1 receptor in the development of acute gout attacks. Rheumatology (Oxford). 2014;53(2):240–9.
Hoffmeister C, Trevisan G, Rossato MF, de Oliveira SM, Gomez MV, Ferreira J. Role of TRPV1 in nociception and edema induced by monosodium urate crystals in rats. Pain. 2011;152(8):1777–88.
Hong J, Qiu J, Wang X, Zhang G. Characteristics of voltage-gated potassium currents in monosodium urate induced gouty arthritis in mice. Inflamm Res. 2020;69(6):589–98.
Qiu J, Xu X, Zhang S, Li G, Zhang G. Modulations of Nav1.8 and Nav1.9 channels in monosodium urate-induced gouty arthritis in mice. Inflammation. 2021;44(4):1405–15.
Sui F, Zhang CB, Yang N, Li LF, Guo SY, Huo HR, et al. Anti-nociceptive mechanism of baicalin involved in intervention of TRPV1 in DRG neurons in vitro. J Ethnopharmacol. 2010;129(3):361–6.
Roh J, Hwang SM, Lee SH, Lee K, Kim YH, Park CK. Functional expression of piezo1 in dorsal root ganglion (DRG) neurons. Int J Mol Sci. 2020;21(11):3834.
Silverman HA, Chen A, Kravatz NL, Chavan SS, Chang EH. Involvement of neural transient receptor potential channels in peripheral inflammation. Front Immunol. 2020;11:590261.
Galindo T, Reyna J, Weyer A. Evidence for transient receptor potential (TRP) channel contribution to arthritis pain and pathogenesis. Pharmaceuticals (Basel). 2018;11(4):105.
Eun SY, Jung SJ, Park YK, Kwak J, Kim SJ, Kim J. Effects of capsaicin on Ca2+ release from the intracellular Ca2+ stores in the dorsal root ganglion cells of adult rats. Biochem Biophys Res Commun. 2001;285(5):1114–20.
Gu Y, Li G, Huang LM. Inflammation induces Epac-protein kinase C alpha and epsilon signaling in TRPV1-mediated hyperalgesia. Pain. 2018;159(11):2383–93.
Acknowledgements
The study was supported by Open Fund of Key Laboratory of Medical Electrophysiology, Ministry of Education, Southwest Medical University.
Author information
Authors and Affiliations
Contributions
GZ designed the study; XX, ZY, SZ performed the experiments and analyzed the date; GZ and GL edited and reviewed the manuscript. All authors revised the manuscript and approved the final view.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Animal experiments were authorized by the Institutional Animal Care and Use Committee of China Pharmaceutical University.
Additional information
Responsible Editor: Jason J. McDougall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, X., Yuan, Z., Zhang, S. et al. Regulation of TRPV1 channel in monosodium urate-induced gouty arthritis in mice. Inflamm. Res. 71, 485–495 (2022). https://doi.org/10.1007/s00011-022-01561-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01561-7