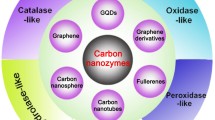
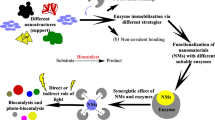
Abstract
Enzymes are essential biological catalysts that can accelerate multiple reactions. Their outstanding catalytic properties make them highly valuable in different research fields and industries including pharmaceutical, sensing, food, and agriculture. However, the catalytic attributes of free enzymes are limited by their poor stability and resistance to harsh conditions. Recently, the conjugation of different enzymes with carbon dots (CDs) has been explored as a novel strategy for tuning their catalytic properties. CDs possess unique and tunable characteristics such as light stability, electron transfer properties, lower toxicity, cost-efficiency, and outstanding biocompatibility; thus, they represent excellent options for the conjugation of different enzymes to improve their stability, selectivity, and catalytic efficiency. Recently, various CDs-based nano-biocatalysts have been successfully prepared with superior performances compared to their free enzymes. Therefore, this review aims to discuss the most recent reported studies in the synthesis of CDs-based nano-biocatalysts providing an overview of current methodologies and recent research applications. Lastly, we delve into the prospects and the future possibilities of such innovative conjugates that entail an exploration of the faced challenges and their untapped potential for various applications.
Graphical Abstract




Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- CDs:
-
Carbon dots
- N-CDs:
-
Nitrogen-doped carbon dots
- GQD:
-
Graphene quantum dots
- g-CNQDs:
-
Graphitic carbon nitride quantum dots
- CQD:
-
Carbon quantum dots
- CNDs:
-
Carbon nanodots
- CPDs:
-
Carbonized polymer dots
- TYR:
-
Tyrosinase
- EDC:
-
1-Ethyl-3-(3′-dimethylaminopropyl)carbodiimide
- NHS:
-
N-Hydroxysuccinimide
- DA:
-
Dopamine
- HRP:
-
Horseradish peroxidase
- NP:
-
Nanoparticle
- PCDs:
-
Phosphate-modified carbon dots
- PPL:
-
Pancreatic lipase
- Tyr:
-
l-Tyrosine methyl ester
References
Wei SC, Lin YW, Chang HT (2020) Carbon dots as artificial peroxidases for analytical applications. J Food Drug Anal 28:558–574. https://doi.org/10.38212/2224-6614.1090
Villalba-Rodríguez AM, Parra-Arroyo L, González-González RB et al (2022) Laccase-assisted biosensing constructs—robust modalities to detect and remove environmental contaminants. Case Stud Chem Environ Eng 5:100180. https://doi.org/10.1016/j.cscee.2022.100180
Kumar L, Bharadvaja N (2019) Chapter 6—Enzymatic bioremediation: a smart tool to fight environmental pollutants. In: Bhatt P (ed) Smart bioremediation technologies. Academic, pp 99–118
Lopez-Cantu DO, González-González RB, Melchor-Martínez EM et al (2022) Enzyme-mimicking capacities of carbon-dots nanozymes: properties, catalytic mechanism, and applications—a review. Int J Biol Macromol 194:676–687. https://doi.org/10.1016/j.ijbiomac.2021.11.112
Lopez-Cantu DO, González-González RB, Sharma A et al (2022) Bioactive material-based nanozymes with multifunctional attributes for biomedicine: expanding antioxidant therapeutics for neuroprotection, cancer, and anti-inflammatory pathologies. Coord Chem Rev 469:214686. https://doi.org/10.1016/j.ccr.2022.214685
Rodrigues RC, Berenguer-Murcia Á, Carballares D et al (2021) Stabilization of enzymes via immobilization: multipoint covalent attachment and other stabilization strategies. Biotechnol Adv 52:107821. https://doi.org/10.1016/j.biotechadv.2021.107821
Rybarczyk A, Smułek W, Grzywaczyk A et al (2023) 3D printed polylactide scaffolding for laccase immobilization to improve enzyme stability and estrogen removal from wastewater. Bioresour Technol 381:129144. https://doi.org/10.1016/j.biortech.2023.129144
Lee M-K, Lee YJ, Kang JY, Lee SH (2021) Strong enzyme immobilization associated by anatase TiO2 sputtered on platinum black nanoclusters to improve sensitivity and long-term stability of electrochemical cholesterol sensor. Sens Actuators B Chem 334:129617. https://doi.org/10.1016/j.snb.2021.129617
Sharma SK, Micic M, Li S et al (2019) Conjugation of carbon dots with β-galactosidase enzyme: surface chemistry and use in biosensing. Molecules 24:3275. https://doi.org/10.3390/molecules24183275
Kalsoom U, Khalid N, Ibrahim A et al (2023) Biocatalytic degradation of reactive blue 221 and direct blue 297 dyes by horseradish peroxidase immobilized on iron oxide nanoparticles with improved kinetic and thermodynamic characteristics. Chemosphere 312:137095. https://doi.org/10.1016/j.chemosphere.2022.137095
Amruth Maroju P, Ganesan R, Ray Dutta J (2023) Biofuel generation from food waste through immobilized enzymes on magnetic nanoparticles. Mater Today Proc 72:62–66. https://doi.org/10.1016/j.matpr.2022.05.555
Xu X, Chen T, Xu L, Lin J (2024) Immobilization of laccase on magnetic nanoparticles for enhanced polymerization of phenols. Enzyme Microb Technol 172:110331. https://doi.org/10.1016/j.enzmictec.2023.110331
Jimenez-Carretero M, Jabalera Y, Sola-Leyva A et al (2023) Nanoassemblies of acetylcholinesterase and β-lactamase immobilized on magnetic nanoparticles as biosensors to detect pollutants in water. Talanta 258:124406. https://doi.org/10.1016/j.talanta.2023.124406
González-González RB, Martínez-Zamudio LY, Hernández JAR et al (2023) Pharmaceutical pollution fingerprinting and waterbodies remediation using waste-derived carbon dots as sustainable advanced nanomaterials. Environ Res 238:117180. https://doi.org/10.1016/j.envres.2023.117180
Ponmudi K, Cherian AR, Varghese A (2023) Chapter 18—Carbon dots as an effective material in enzyme immobilization for sensing applications. In: Kailasa SK, Hussain CM (eds) Carbon dots in analytical chemistry. Elsevier, pp 241–253
Wu H, Mu W (2022) Application prospects and opportunities of inorganic nanomaterials for enzyme immobilization in the food-processing industry. Curr Opin Food Sci 47:100909. https://doi.org/10.1016/j.cofs.2022.100909
Adeel M, Bilal M, Rasheed T et al (2018) Graphene and graphene oxide: functionalization and nano-bio-catalytic system for enzyme immobilization and biotechnological perspective. Int J Biol Macromol 120:1430–1440. https://doi.org/10.1016/j.ijbiomac.2018.09.144
Bilal M, Rashid EU, Munawar J et al (2023) Magnetic metal-organic frameworks immobilized enzyme-based nano-biocatalytic systems for sustainable biotechnology. Int J Biol Macromol 237:123968. https://doi.org/10.1016/j.ijbiomac.2023.123968
González-González RB, Sharma A, Parra-Saldívar R et al (2022) Decontamination of emerging pharmaceutical pollutants using carbon-dots as robust materials. J Hazard Mater 423:127145. https://doi.org/10.1016/j.jhazmat.2021.127145
González-González RB, Morales-Murillo MB, Martínez-Prado MA et al (2022) Carbon dots-based nanomaterials for fluorescent sensing of toxic elements in environmental samples: strategies for enhanced performance. Chemosphere 300:134515. https://doi.org/10.1016/j.chemosphere.2022.134515
Mansuriya BD, Altintas Z (2021) Carbon dots: classification, properties, synthesis, characterization, and applications in health care—an updated review (2018–2021). Nanomaterials 11:2525. https://doi.org/10.3390/nano11102525
Li S, Li L, Tu H et al (2021) The development of carbon dots: from the perspective of materials chemistry. Mater Today 51:188–207. https://doi.org/10.1016/j.mattod.2021.07.028
González-González RB, González LT, Madou M et al (2022) Synthesis, purification, and characterization of carbon dots from non-activated and activated pyrolytic carbon black. Nanomaterials 12:298. https://doi.org/10.3390/nano12030298
Li X, Zhang S, Kulinich SA et al (2014) Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci Rep 4:4976. https://doi.org/10.1038/srep04976
Siddique AB, Hossain SM, Pramanick AK, Ray M (2021) Excitation dependence and independence of photoluminescence in carbon dots and graphene quantum dots: insights into the mechanism of emission. Nanoscale 13:16662–16671. https://doi.org/10.1039/D1NR04301C
Srivastava I, Khamo JS, Pandit S et al (2019) Influence of electron acceptor and electron donor on the photophysical properties of carbon dots: a comparative investigation at the bulk-state and single-particle level. Adv Funct Mater 29:1902466. https://doi.org/10.1002/adfm.201902466
Gao W, Zhang S, Wang G et al (2022) A review on mechanism, applications and influencing factors of carbon quantum dots based photocatalysis. Ceram Int 48:35986–35999. https://doi.org/10.1016/j.ceramint.2022.10.116
Gallareta-Olivares G, Rivas-Sanchez A, Cruz-Cruz A et al (2023) Metal-doped carbon dots as robust nanomaterials for the monitoring and degradation of water pollutants. Chemosphere 312:137190. https://doi.org/10.1016/j.chemosphere.2022.137190
Liu J, Li R, Yang B (2020) Carbon dots: a new type of carbon-based nanomaterial with wide applications. ACS Cent Sci 6:2179–2195. https://doi.org/10.1021/acscentsci.0c01306
Kurian M, Paul A (2021) Recent trends in the use of green sources for carbon dot synthesis—a short review. Carbon Trends 3:100032. https://doi.org/10.1016/j.cartre.2021.100032
Cruz-Cruz A, Gallareta-Olivares G, Rivas-Sanchez A et al (2022) Recent advances in carbon dots based biocatalysts for degrading organic pollutants. Curr Pollut Rep 8:384–394. https://doi.org/10.1007/s40726-022-00228-5
González-González RB, Parra-Saldívar R, Ramirez-Mendoza RA, Iqbal HMN (2022) Carbon dots as a new fluorescent nanomaterial with switchable sensing potential and its sustainable deployment for metal sensing applications. Mater Lett 309:131372. https://doi.org/10.1016/j.matlet.2021.131372
Shi W, Han Q, Wu J et al (2022) Synthesis mechanisms, structural models, and photothermal therapy applications of top-down carbon dots from carbon powder, graphite, graphene, and carbon nanotubes. Int J Mol Sci 23:1456. https://doi.org/10.3390/ijms23031456
Limosani F, Bauer EM, Cecchetti D et al (2021) Top-down N-doped carbon quantum dots for multiple purposes: heavy metal detection and intracellular fluorescence. Nanomaterials 11:2249. https://doi.org/10.3390/nano11092249
Yew YT, Loo AH, Sofer Z et al (2017) Coke-derived graphene quantum dots as fluorescence nanoquencher in DNA detection. Appl Mater Today 7:138–143. https://doi.org/10.1016/j.apmt.2017.01.002
Yao B, Huang H, Liu Y, Kang Z (2019) Carbon dots: a small conundrum. Trends Chem 1:235–246. https://doi.org/10.1016/j.trechm.2019.02.003
Frank BP, Sigmon LR, Deline AR et al (2020) Photochemical transformations of carbon dots in aqueous environments. Environ Sci Technol 54:4160–4170. https://doi.org/10.1021/acs.est.9b07437
Ricardo S, del Valle Martínez de Yuso M, Algarra M et al (2020) Comparative life cycle assessment of bottom-up synthesis routes for carbon dots derived from citric acid and urea. J Clean Prod 254:120080. https://doi.org/10.1016/j.jclepro.2020.120080
Gusain D, Renuka N, Guldhe A, Bux F (2021) Use of microalgal lipids and carbohydrates for the synthesis of carbon dots via hydrothermal microwave treatment. Inorg Chem Commun 134:109021. https://doi.org/10.1016/j.inoche.2021.109021
Rabiee N, Iravani S, Varma RS (2022) Biowaste-derived carbon dots: a perspective on biomedical potentials. Molecules 27:6186. https://doi.org/10.3390/molecules27196186
Cárdenas-Alcaide MF, González-González RB, Villalba-Rodríguez AM et al (2023) Nanofabrication and characterization of green-emitting N-doped carbon dots derived from pulp-free lemon juice extract. Nanofabrication. https://doi.org/10.37819/nanofab.008.299
Jorns M, Pappas D (2021) A review of fluorescent carbon dots, their synthesis, physical and chemical characteristics, and applications. Nanomaterials 11:1448. https://doi.org/10.3390/nano11061448
Feng Z, Adolfsson KH, Xu Y et al (2021) Carbon dot/polymer nanocomposites: from green synthesis to energy, environmental and biomedical applications. Sustain Mater Technol 29:e00304. https://doi.org/10.1016/j.susmat.2021.e00304
Chahal S, Macairan J-R, Yousefi N et al (2021) Green synthesis of carbon dots and their applications. RSC Adv 11:25354–25363. https://doi.org/10.1039/D1RA04718C
Mohamad NR, Marzuki NHC, Buang NA et al (2015) An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip 29:205–220. https://doi.org/10.1080/13102818.2015.1008192
Sharifi M, Sohrabi MJ, Hosseinali SH et al (2020) Enzyme immobilization onto the nanomaterials: application in enzyme stability and prodrug-activated cancer therapy. Int J Biol Macromol 143:665–676. https://doi.org/10.1016/j.ijbiomac.2019.12.064
Thangaraj B, Solomon PR (2019) Immobilization of lipases—a review. Part I: Enzyme immobilization. ChemBioEng Rev 6:157–166. https://doi.org/10.1002/cben.201900016
Tang Z, Jiang K, Sun S et al (2019) A conjugated carbon-dot–tyrosinase bioprobe for highly selective and sensitive detection of dopamine. Analyst 144:468–473. https://doi.org/10.1039/C8AN01659C
Das K, Maiti S, Das PK (2014) Probing enzyme location in water-in-oil microemulsion using enzyme-carbon dot conjugates. Langmuir 30:2448–2459. https://doi.org/10.1021/la403835h
Li H, Guo S, Li C et al (2015) Tuning laccase catalytic activity with phosphate functionalized carbon dots by visible light. ACS Appl Mater Interfaces 7:10004–10012. https://doi.org/10.1021/acsami.5b02386
Li H, Kong W, Liu J et al (2014) Carbon dots for photoswitching enzyme catalytic activity. J Mater Chem B 2:5652–5658. https://doi.org/10.1039/C4TB00705K
Chen Q, Man H, Zhu L et al (2020) Enhanced plant antioxidant capacity and biodegradation of phenol by immobilizing peroxidase on amphoteric nitrogen-doped carbon dots. Catal Commun 134:105847. https://doi.org/10.1016/j.catcom.2019.105847
Muthurasu A, Ganesh V (2014) Horseradish peroxidase enzyme immobilized graphene quantum dots as electrochemical biosensors. Appl Biochem Biotechnol 174:945–959. https://doi.org/10.1007/s12010-014-1019-7
Razmi H, Mohammad-Rezaei R (2013) Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: application to sensitive glucose determination. Biosens Bioelectron 41:498–504. https://doi.org/10.1016/j.bios.2012.09.009
Baluta S, Lesiak A, Cabaj J (2018) Graphene quantum dots-based electrochemical biosensor for catecholamine neurotransmitters detection. Electroanalysis 30:1781–1790. https://doi.org/10.1002/elan.201700825
Agrawal DC, Yadav A, Kesarwani R et al (2020) Immobilization of fenugreek β-amylase onto functionalized graphene quantum dots (GQDs) using Box-Behnken design: its biochemical, thermodynamic and kinetic studies. Int J Biol Macromol 144:170–182. https://doi.org/10.1016/j.ijbiomac.2019.12.033
Karaca Açarı İ, Dik G, Bakar B et al (2023) Immobilization of α-amylase onto quantum dots prepared from Hypericum perforatum L. flowers and Hypericum capitatum seeds: its physicochemical and biochemical characterization. Top Catal 66:563–576. https://doi.org/10.1007/s11244-022-01699-y
Ren Y, Rivera JG, He L et al (2011) Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol 11:63. https://doi.org/10.1186/1472-6750-11-63
Villalba-Rodríguez AM, Martínez-Zamudio LY, Martínez SAH et al (2023) Nanomaterial constructs for catalytic applications in biomedicine: nanobiocatalysts and nanozymes. Top Catal 66:707–722. https://doi.org/10.1007/s11244-022-01766-4
Zhu H, Wang E, Li J, Wang J (2018) L-Tyrosine methyl ester-stabilized carbon dots as fluorescent probes for the assays of biothiols. Anal Chim Acta 1006:83–89. https://doi.org/10.1016/j.aca.2017.12.014
Sangubotla R, Kim J (2021) Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater Sci Eng C 122:111916. https://doi.org/10.1016/j.msec.2021.111916
Ji H, Zhou F, Gu J et al (2016) Nitrogen-doped carbon dots as a new substrate for sensitive glucose determination. Sensors (Switzerland) 16(5):630. https://doi.org/10.3390/s16050630
Zhao D, Li X, Xu M et al (2023) Preparations of antibacterial yellow-green-fluorescent carbon dots and carbon dots-lysozyme complex and their applications in bacterial imaging and bacteria/biofilm inhibition/clearance. Int J Biol Macromol 231:123303. https://doi.org/10.1016/j.ijbiomac.2023.123303
Singh A, Verma A, Singh R et al (2020) Combination therapy of biogenic C-dots and lysozyme for enhanced antibacterial and antibiofilm activity. Nanotechnology 32:85104. https://doi.org/10.1088/1361-6528/abc2ed
Zhao D, Zhang R, Xu M et al (2022) A novel quaternized carbon dot–papain complex for the double-target anti-biofilm activity and visualization-ratio fluorescence dual-mode detection of H2O2. Mater Chem Front 6:2848–2858. https://doi.org/10.1039/D2QM00347C
Gaviria MI, Barrientos K, Arango JP et al (2022) Highly sensitive fluorescent biosensor based on acetylcholinesterase and carbon dots-graphene oxide quenching test for analytical and commercial organophosphate pesticide detection. Front Environ Sci 10:825112. https://doi.org/10.3389/fenvs.2022.825112
Han E, Yang Y, He Z et al (2015) Development of tyrosinase biosensor based on quantum dots/chitosan nanocomposite for detection of phenolic compounds. Anal Biochem 486:102–106. https://doi.org/10.1016/j.ab.2015.07.001
Liu L, Anwar S, Ding H et al (2019) Electrochemical sensor based on F,N-doped carbon dots decorated laccase for detection of catechol. J Electroanal Chem 840:84–92. https://doi.org/10.1016/j.jelechem.2019.03.071
Krishnaiah P, Atchudan R, Perumal S et al (2022) Utilization of waste biomass of Poa pratensis for green synthesis of n-doped carbon dots and its application in detection of Mn2+ and Fe3+. Chemosphere 286:131764. https://doi.org/10.1016/j.chemosphere.2021.131764
Essner JB, Kist JA, Polo-Parada L, Baker GA (2018) Artifacts and errors associated with the ubiquitous presence of fluorescent impurities in carbon nanodots. Chem Mater 30:1878–1887. https://doi.org/10.1021/acs.chemmater.7b04446
Mintz KJ, Bartoli M, Rovere M et al (2021) A deep investigation into the structure of carbon dots. Carbon N Y 173:433–447. https://doi.org/10.1016/j.carbon.2020.11.017
Acknowledgements
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) and Tecnologico de Monterrey, Mexico under Sistema Nacional de Investigadores (SNI) program awarded to Roberto Parra-Saldívar (CVU: 35753).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cruz-Cruz, A., Rivas-Sanchez, A., González-González, R.B. et al. Tuning Catalytic Attributes of Enzymes by Conjugation with Functionalized Carbon Dots. Top Catal (2024). https://doi.org/10.1007/s11244-024-01911-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-024-01911-1