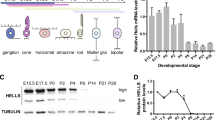
Abstract
To assess whether retinoblastoma formation is associated with the expression of high mobility group (HMG) A2 protein, a transcription factor that is highly expressed during embryogenesis and completely repressed in normal adult tissues, we performed Northern and Western blots and RT-PCR analyses, and immunohistochemistry to test for HMGA2 expression. We used established retinoblastoma cell lines in tumors grown in nude mice and clinical retinoblastoma specimens, and contrasted these tumors with normal embryonic and adult retina. Adenoviral-mediated antisense experiments were conducted on the retinoblastoma cell lines to suppress HMGA2 expression and determine if cell proliferation is HMGA2-dependent. We also transfected a retinoblastoma cell line to identify cis-regulatory elements and transcription initiation sites on the HMGA2 gene promoter. HMGA2 gene expression was silenced in terminally differentiated retina of 6-wk-old mice, but it was detected in retina of a 13.5-d postcoitum embryo. Reactivation of HMGA2 gene expression was observed in the retinoblastoma cell lines Y79, WERI-Rb1, and TOTL-1, in tumors derived from some of these cells propagated in nude mice, and in a high frequency of retinoblastomas excised from human patients. This suggests that expression of HMGA2 gene in retinoblastoma cells involves a derepression process. By using an antisense approach to block HMGA2 expression, we observed a decrease in the number of proliferating retinoblastoma cells. As a 1st step toward understanding HMGA2 gene reactivation in retinoblastoma, we mapped the 2 transcription initiation sites and associated positive regulatory elements within the WERI-Rb1 cells. Our discovery of derepression of HMGA2 gene expression in retinoblastoma provides the 1st evidence that this protein might contribute to neoplastic transformation of retina cells.







Similar content being viewed by others
References
Albert DA, Jakobiec FA (eds). (1994) Principles and practice of ophthalmology. Sauders, Philadelphia.
Friend SH et al. (1986) A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323:643–6.
Lee WH et al. (1987) Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science 235:1394–9.
Fung YK et al. (1987) Structural evidence for the authenticity of the human retinoblastoma gene. Science 236:1657–61.
Goodwin G. (1998) Molecules in focus: the high mobility group protein, HMGI-C. Int J Biochem. Cell Biol. 30:761–6.
Zhou X, Chada K. (1998) HMGI family proteins: architectural transcription factors in mammalian development and cancer. Keio J. Med. 47:73–7.
Liu F, Chau KY, Arlotta P, Ono SJ. (2001) The HMG I proteins: dynamic roles in gene activation, development, and tumorigenesis. Immunol. Res. 24:13–29.
Reeves R. (2001) Molecular biology of HMGA proteins: hubs of nuclear function. Gene 277:63–81.
Fedele M et al. (2001) Role of the high mobility group A proteins in human lipomas. Carcinogenesis 22:1583–91.
Wolffe A. (1994) Architectural transcription factors. Science 264:1100–1.
Mantovani F et al. (1998) NF-κB mediated transcriptional activation is enhanced by the architectural factor HMGI-C. Nucleic Acids Res. 26:1433–9.
Noro B et al. (2003) Molecular dissection of the architectural transcription factor HMGA2. Biochemistry 42:4569–77.
Zhou X et al. (1995) Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature 376:771–4.
Zhou X et al. (1996) Genomic structure and expression of the murine HMGI-C gene. Nucleic Acids Res. 24:4071–7.
Rogalla P et al. (1996) HMGI-C expression patterns in human tissues. implications for the genesis of frequent mesenchymal tumors. Am. J. Pathol. 149:775–9.
Hirning-Folz U et al. (1998) The expression pattern of the HMGIC gene during development. Gene Chrom. Cancer 23:771–4.
Chieffi P et al. (2002) HMGA1 and HMGA2 protein expression in mouse spermatogenesis. Oncogene 21:3644–50.
Giancotti V et al. (1985) Changes in nuclear proteins following transformation of rat thyroid epithelial cells by a murine sarcoma retrovirus. Cancer Res. 45:6051–7.
Giancotti V et al. (1987) Elevated levels of a specific class of nuclear phospho-proteins in cells transformed with v-ras and v-mos oncogenes and by co-transfection with c-myc and polyma middle T genes. EMBO J. 6:1981–7.
Giancotti V et al. (1991) Comparison of multiple forms of the high mobility group I proteins in rodent and human cells. Identification of the human high mobility group I-C protein. Eur. J. Biochem. 198:211–6.
Manfioletti G et al. (1991) cDNA cloning of the HMGI-C phosphoprotein, a nuclear protein associated with neoplastic and undifferentiated phenotypes. Nucleic Acids Res. 19:6793–7.
Patel UA et al. (1994) Expression and cDNA cloning of human HMGI-C phos-phoprotein. Biochem. Biophys. Res. Commun. 201:63–70.
Rogalla P et al. (1997) Expression of HMGI-C, a member of the high mobility group protein family, in a subset of breast cancers: relationship to histologic grade. Mol. Carcinog. 19:153–6.
Rogalla P et al. (1998) HMGIC expression patterns in non-small lung cancer and surrounding tissue. Anticancer Res. 18:3327–30.
Finelli P et al. (2002) The High Mobility Group A2 gene is amplified and overexpressed in human prolactinomas. Cancer Res. 62:2398–405.
Wunderlich V, Bottger M. (1997) High-mobility-group proteins and cancer: an emerging link. J. Cancer Res. Clin. Oncol. 123:133–40.
Wood LJ, Maher JF, Bunton TE, Resar LM. (2000) The oncogenic properties of the HMG-I gene family. Cancer Res. 60:4256–61.
Fedele M et al. (2002) Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene 21:3190–8.
Schoenmakers EFPM et al. (1995) Recurrent rearrangements in the high mobility group protein gene, HMGI-C, in benign mesenchymal tumours. Nature Genet. 10:436–44.
Ashar HR et al. (1995) Disruption of the architectural factor HMGI-C: DNA-binding AT hook motifs fused in lipomas to distinct transcriptional regulatory domains. Cell. 82:57–65.
Kazmierczak B et al. (1996) HMG I-C rearrangements as the molecular basis for the majority of pulmonary chondroid hamartomas: a survey of 30 tumors. Oncogene 12:515–21.
Berner JM et al. (1997) HMGIC, the gene for an architectural transcription factor, is amplified and rearranged in a subset of human sarcomas. Oncogene 14:2935–41.
Pedeutour F et al. (2000) Dysregulation of HMGIC in a uterine lipoleiomyoma with a complex rearrangement including chromosomes 7, 12, and 14. Genes Chrom. Cancer 27:209–15.
Finelli P et al. (2002) The High Mobility Group A2 gene is amplified and overex-pressed in human prolactinomas. Cancer Res. 62:2398–405.
Fedele M et al. (1998) Truncated and chimeric HMGI-C genes induce neoplastic transformation of NIH3T3 murine fibroblasts. Oncogene 17:413–8.
Battista S et al. (1999) The expression of a truncated HMGI-C gene induces gigantism associated with lipomatosis. Cancer Res. 59:4793–7.
Arlotta P et al. (2000) Transgenic mice expressing a truncated form of the high mobility group i-c protein develop adiposity and an abnormally high prevalence of lipomas. J. Biol. Chem. 275:14394–400.
Chau KY et al. (2000) The architectural transcription factor high mobility group I(Y) participates in photoreceptor-specific gene expression. J Neurosci. 20:7317–24.
Sasabe T et al. (1991) Cyclic nucleotides and differentiation of retinoblastoma. Folia Ophthalmol. 42:1200–6.
Vanhamme L, Szpirer C. (1988) Transforming activity of the human mammary line HBL100 DNA is associated with SV40 T antigen genetic information integrated in its genome. Carcinogenesis 9:653–5.
Johnson KR et al. (1988) Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y). J. Biol. Chem. 263:18338–42.
Chiappetta G et al. (1996) High level expression of the HMGI(Y) gene during embryonic development. Oncogene 13:2439–46.
Scala S et al. (2000) Adenovirus-mediated suppression of HMGI(Y) protein synthesis as potential therapy of human malignant neoplasias. Proc. Natl. Acad. Sci. U.S.A. 97:4256–61.
Chau KY, Ono SJ. (1999) Gene transfer into retinoblastoma cells. Biotechniques 26:444–6.
Chau K et al. (1999) A novel downstream positive regulatory element mediating transcription of the human high mobility group (HMG) I-C gene. FEBS Lett. 457:429–36.
Berlingieri MT et al. (1995) Inhibition of HMGI-C protein synthesis suppresses retrovirally induced neoplastic transformation of rat thyroid cells. Mol. Cell Biol. 15:1545–53.
White JB, Taylor RE, Pittler SJ. (2001) Reproducible high efficiency gene transfer into Y79 retinoblastoma cells using adenofection. J. Neurosci. Methods 106:1–7.
Chau K-Y et al. (1995) The gene for the human architectural transcription factor HMGI-C consists of five exons each coding for a distinct functional element. Nucleic Acids Res. 23:4262–6.
Prestridge DS. (1991) Signal scan: a computer program that scan DNA sequences for eukaryotic transcriptional elements. CABIOS 7:203–6.
Ishii S et al. (1985) Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc. Natl. Acad. Sci. U.S.A. 82:4920–4.
Friedmann M et al. (1993) Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res. 21:4259–67.
Acknowledgments
We thank Drs Gilbert Jay, C Kathleen Dorey, Patricia Pearson, and other members of our laboratory, in particular: Drs Andrea Keane-Myers, Dai Miyazaki, Paola Arlotta, Takao Nakamura, and Jason Kreisberg who provided advice and help with the manuscript. We also thank Dr Robert Weinberg, Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology, for the gifts of Y79, WERI-Rb1, and OSH50T cells; and the Tissue Culture Core Facility of the Lombardi Cancer Center for HBL–100 cells. This work was supported by National Institute of General Medical Science grant RO1 GM49661, the Lucille P Markey Charitable Trust, the Research to Prevent Blindness Foundation, and the “Associazione Italiana per la Ricerca sul Cancro.” GM was supported by “Fondazione Italiana per la Ricerca sul Cancro” while he was a Visiting Scientist of the Ono Lab. SJO was a JSPS Visiting Professor at Kyoto University during the preparation of this manuscript. K-YC was a Fellow of the Research to Prevent Blindness, America Fund (PD97054). SM was supported by the Massachusetts Lions Eye Research Fund and Gifts to the Retina Genetics Fund, Massachusetts Eye & Ear Infirmary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chau, KY., Manfioletti, G., Cheung-Chau, KW. et al. Derepression of HMGA2 Gene Expression in Retinoblastoma Is Associated with Cell Proliferation. Mol Med 9, 154–165 (2003). https://doi.org/10.1007/BF03402180
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402180