Abstract
Background
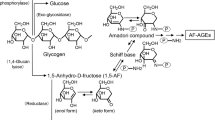
The advanced stage of the Maillard reaction that leads to the formation of advanced glycation end-products (AGEs) plays an important role in the pathogenesis of angiopathy in diabetic patients and in the aging process. Recently, it has been proposed that the intermediates contributing to AGE formation include dicarbonyl intermediates such as glyoxal, methylglyoxal, and 3-deoxyglucosone (3-DG). In the present study, we developed a novel, non-carboxymethyllysine (CML) anti-AGE antibody that recognizes serum proteins and peptides modified by 3-DG in vivo.
Materials and Methods
AGE-modified serum albumins were prepared by incubation of rabbit serum albumin with 3-DG or D-glucose. After immunization of rabbits, anti-AGE antisera were subjected to affinity chromatography on a Sepharose 4B column coupled with CML-BSA, or AGE-BSA created by incubation with 3-DG (AGE-6) or D-glucose (AGE-1). The AGE-Ab-6 and AGE-Ab-1 thus obtained was used to investigate AGEs in serum from diabetic patients on hemodialysis.
Results
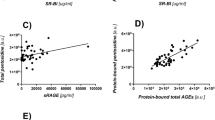
Characterization of the novel AGE-Ab-6 obtained by immunoaffinity chromatography was performed with a competitive ELISA and immunoblot analysis. This antibody specifically cross-reacted with proteins modified by 3-DG. AGE-6 was detected in diabetic serum as three peaks with apparent molecular weights of 200, 1.15, and 0.85 kD, while AGE-1 was detected as four peaks with apparent molecular weights of 200, 65, 1.15, and 0.85 kD.
Conclusion
This study provides new data on the pathways of AGE formation from 3-DG and methods for the immunochemical detection of AGEs. We also provide immunochemical evidence for the existence of six distinct AGEs in vivo among the AGE-modified proteins and peptides in the serum of diabetic patients on hemodialysis.






Similar content being viewed by others
References
Bucala R, Cerami A. (1992) Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv. Pharmcol. 23: 1–34.
Vlassara H, Bucala R, Striker L. (1994) Pathogenic effects of AGEs: biochemical, biologic, and clinical implications for diabetes and aging. Lab. Invest. 70: 138–151.
Brownlee M. (1995) Advanced protein glycosylation in diabetes and aging. Ann. Rev. Med. 46: 223–234.
Vlassara H. (1997) Recent progress in advanced glycation end products and diabetic complications. Diabetes 46: S19–S25.
Vitek MP, Bhattacharya K, Glendening JM, et al. (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 91: 4766–4770.
Smith MA, Taneda S, Richey PL, et al. (1994) Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc. Natl. Acad. Sci. USA 91: 5710–5714.
Yan SD, Chen X, Schmidt AM, et al. (1994) Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc. Natl. Acad. Sci. USA 91: 7787–7791.
Sasaki N, Fukatsu R, Tsuzuki K, et al. (1998) Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am. J. Pathol. 153: 1149–1155.
Castellani, R, Smith MA, Richey PJ, Petty G. (1996) Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 737: 195–200.
Shibata N, Hirano A, Kato S, et al. (1999) Advanced glycation endproducts are deposited in neuronal hyaline inclusions: a study of familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Acta Neuropathol. 97: 240–246.
Chou SM, Wang HS, Taniguchi A, Bucala R. (1998) Advanced glycation end products in neurofilament conglomeration of motoneurons in familial and sporadic amyotrophic lateral sclerosis. Mol. Med. 4: 324–332.
Konishi Y, Hayase F, Kato H. (1994) Novel imidazolone compound by the advanced Maillard reaction of 3-deoxyglucosone and arginine residues in proteins. Biosci. Biotech. Biochem. 58: 1953–1955.
Hayase F, Konishi Y, Kato H. (1995) Identification of the modified structure of arginine residues in proteins with 3-deoxyglucosone, a Maillard reaction intermediate. Biosci. Biotech. Biochem. 59: 1407–1411.
Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. (1996) The advanced glycation end product, N-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 271: 9982–9986.
Wells-Knecht KK, Lyons TJ, McCance DR, Thorpe SR, Feather MS, Baynes JW. (1994) 3-Deoxyfructose concentrations are increased in human plasma and urine in diabetes. Diabetes 43: 1152–1156.
Nagaraj RH, Sady C. (1996) The presence of a glucose-derived Maillard reaction product in the human lens. FEBS Lett. 382: 234–238.
Al-Abed Y, Kapurniotu A, Bucala R. (1999) Advanced glycation end products; detection and reversal. Methods Enzymol. 309: 152–172.
Lal S, Szwergold BS, Taylor AH, et al. (1995) Metabolism of fructose-3-phosphate in the diabetic rat lens. Arch. Biochem. Biophys. 318: 191–199.
Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. (1991) Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J. Biol. Chem. 266: 11654–11660.
Niwa T. (1999) 3-Deoxyglucosone: metabolism, analysis, biological activity, and clinical implication. J. Chromatogr. B 731: 23–36.
Niwa T, Katsuzaki T, Momoi T, et al. (1996) Modification of beta 2m with advanced glycation end products as observed in dialysis-related amyloidosis by 3-DG. accumulating in uremic serum. Kidney Int. 49: 861–867.
Odani H, Shinzato T, Matsumoto Y, Usami J, Maeda K. (1999) Increase in three α, β-dicarbonyl compound levels in human uremic plasma: specific in vivo determination of intermediates in advanced Maillard reaction. Biochem. Biophys. Res. Commun. 256: 89–93.
Khadem HE, Horton D, Meshreki MH, Nashed MA. (1971) New route for the synthesis of 3-deoxyaldos-2-uloses. Carbohyd. Res. 17: 183–192.
Makita Z, Vlassara H, Cerami A, Bucala R. (1992) Immunochemical detection of advanced glycosylation end products in vivo. J. Biol. Chem. 267: 5133–5138.
Takeuchi M, Makita Z, Yanagisawa K, Kameda Y, Koike T. (1999) Detection of noncarboxymethyllysine and carboxymethyllysine advanced glycation end products (AGE) in serum of diabetic patients. Mol. Med. 5: 393–405.
Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. (2000) Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol. Med. 6: 114–125.
Ikeda K, Higashi T, Sano H, et al. (1996) N-(carboxymethyl) lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry 35: 8075–8083.
Ahmed MU, Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. (1997) N-(Carboxyethyl)lysine, a product of the chemical modification of proteins by methyglyoxal, increases with age in human lens proteins. Biochem. J. 324: 565–570.
Takeuchi M, Bucala R, Suzuki T, et al. (2000) Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol. 59: 1094–1105.
Brinkmann-Frye E, Degenhardt TP, Thorpe SR, Baynes JW. (1998) Role of the Maillard reaction in aging of tissue proteins: advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J. Biol. Chem. 273: 18714–18719.
Degenhardt TP, Thorpe SR, Baynes JW. (1998) Chemical modification of proteins by methylglyoxal. Cell. Mol. Biol. 44: 1139–1145.
Tessier F, Obrenovich M, Monnier VM. (1999) Structure and mechanism of formation of human lens fluorophore LM-1: relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 274: 20796–20804.
Chellan P, Nagaraj RH. (1999) Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch. Biochem. Biophys. 368: 98–104.
Baynes JW, Thorpe SR. (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48: 1–9.
Uchida K. (2000) Role of reactive aldehyde in cardiovascular diseases. Free Rad. Biol. Med. 28: 1685–1696.
Baynes JW, Thorpe SR. (2000) Glycoxidation and lipoxidation in atherogenesis. Free Rad. Biol. Med. 28: 1708–1716.
Wells-Knecht KJ, Zyzak DV, Litchfield SR, Thorpe SR, Baynes JW. (1995) Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 34: 3702–3709.
Wells-Knecht MC, Thorpe SR, Baynes JW. (1995) Pathways of formation of glycoxidative products during glycation of collagen. Biochemistry 34: 15134–15141.
Acknowledgments
These studies were supported in part by a Grant-in-Aid for Scientific Research (#13470197) to Masayoshi Takeuchi from the Japanese Ministry of Education, Science, Sports, and Culture, by a research grant to Masayoshi Takeuchi from Japan Foundation for Aging and Health, by a research grant to Masayoshi Takeuchi from the Hokuriku University, by a research foundation (#12HIAC124) to Masayoshi Takeuchi from the Hokuriku Industrial Advancement Center, and by the diabetes research foundation (#1-2000-263) to Soroku Yagihashi from Juvenile Diabetes Foundation International.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributed by R. Bucala.
Rights and permissions
About this article
Cite this article
Takeuchi, M., Yanase, Y., Matsuura, N. et al. Immunological Detection of a Novel Advanced Glycation End-Product. Mol Med 7, 783–791 (2001). https://doi.org/10.1007/BF03401969
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401969