Abstract
Experiments were carried out to investigate the contribution of ADP-glucose pyrophosphorylase and the plastid phosphoglucosemutase to the control of starch synthesis. Mutants ofArabidopsis thaliana (L.) Heyhn. were constructed with 50% and 7% of the wild-type adenosine 5′-diphosphoglucose pyrophosphorylase (ADPGlc-PPase), or 50% and null plastid phosphoglucomutase (PGM). The changes in the steady-state rates of sucrose synthesis, starch synthesis and CO2 fixation were measured in saturating CO2 in low (75 μmol·m−2·s−1) and high (600 μmol·m−2·s−1) irradiance. In low irradiance, a 50% decrease of PGM had no significant effect on fluxes, while a 50% and 93% decrease of ADPGlc-PPase led to a 23% and 74% inhibition of starch synthesis. Decreased ADPGlc-PPase led to an increase of hexose phosphates, triose phosphates and fructose-1,6-bisphosphate. Fixation of CO2 was not inhibited because the inhibition of starch synthesis was matched by a stimulation of sucrose synthesis. In high irradiance, a 50% decrease of PGM led to a 20% inhibition of starch synthesis. A 50% and 93% decrease of ADPGlc-PPase led to a 39% and 90% inhibition of starch synthesis. Sucrose synthesis was also inhibited, and the rate of photosynthesis was decreased. Decreased ADPGlc-PPase led to an increase of hexose phosphates but triose phosphates and fructose-1,6-bisphosphate did not increase. These results are used to estimate flux-control coefficients for these enzymes for starch synthesis. Firstly, the flux to starch is only controlled by ADPGlc-PPase in low irradiance, but control is redistributed to other enzymes in the pathway when a rapid flux is imposed, e.g. in high irradiance and CO2. Secondly, reducing the rate of starch synthesis by decreasing the activity of enzymes in this pathway does not always lead to a compensating increase in the rate of sucrose synthesis. Thirdly, decreasing the activity of an enzyme by a factor of two compared to the remainder of the pathway often leads to it exerting very considerable control. Fourthly, each enzyme starts to exert considerable control when only a fraction of its Vmax activity is being utilised in vivo, for example the maximum flux at ADPGlc-PPase never exceeded 20% of the Vmax activity. The summation theory is also applied to check whether additional major control sites are required. In low irradiance, the efficiency of light harvesting will exert considerable control over the rate of starch synthesis.
Similar content being viewed by others
Abbreviations
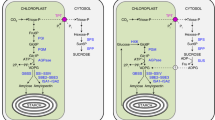
- ADPGlc-PPase:
-
adenosine 5′-diphosphate-glucose pyrophosphorylase
- Fru1,6bisP:
-
fructose-1,6-bisphosphate
- Fru2,6bisP:
-
fructose-2,6-bisphosphate
- Fru6P:
-
fructose-6-phosphate
- Glc1P:
-
glucose-1-phosphate
- Glc6P:
-
glucose-6-phosphate
- PGA:
-
glycerate-3-phosphate
- PGI:
-
phosphoglucose isomerase
- PGM:
-
phosphoglucomutase
- Pi:
-
inorganic phosphate
References
Ball, J.T., Woodrow, I.E., Berry, J.A. (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Progress in photosynthesis research, vol. 3, pp. 521–524, Biggins, J., ed., Nijhoff, Dordrecht
Bassham, J.A., Krause, G.H. (1969) Free energy changes and metabolic regulation in steady state photosynthetic metabolism. Biochim. Biophys. Acta189, 207–221
Bergmeyer, H.U. (1974) Methods of enzymatic analysis. VCH, Weinheim
Caspar, T., Huber, S.C., Somerville, C. (1986) Alterations in growth, photosynthesis and respiration in a starch mutant ofArabidopsis thaliana (L.) Heynh. deficient in chloroplast phosphoglucomutase activity. Plant Physiol.79, 1–7
Caspar, T.M., Lin, T.-P., Monroe, J., Bernhard, W., Spilatro, S., Preiss, J., Somerville, C.R. (1989) Altered regulation of β-amylase activity in mutants ofArabidopsis with lesions in starch metabolism. Proc. Natl. Acad. Sci USA,86, 5830–5833
Dietz, K.-J. (1985) A possible rate-limiting function of chloroplast hexose monophosphate isomerase in starch synthesis in leaves. Biochim. Biophys. Acta839, 240–248
Furbank, R.T., Foyer, C.H., Walker, D.A. (1987) Regulation of photosynthesis in isolated spinach chloroplasts during orthophosphate limitation. Biochim. Biophys. Acta723, 400–409
Gardemann, A., Schimkat, D., Heldt, H.W. (1986) Control of CO2 fixation. Regulation of stromal fructose-1,6-bisphosphatase in spinach by pH and Mg2+ concentrations. Planta168, 536–545
Gerhardt, R., Stitt, M., Heldt, H.W. (1987) Subcellular metabolite levels in spinach leaves. Regulation of sucrose synthesis during diurnal alterations in photosynthesis. Plant Physiol.83, 339–407
Groen, A.K., Wanders, R.T.A., Westerhof, H.U., van der Meer, R., Tager, T.M. (1982) Quantification of the contribution of various steps to the control of mitochondrial respiration. J. Biol. Chem.257, 2754–2757
Heinrich, R., Rapoport, T.A. (1974) A linear steady-state treatment of enzymatic chains. Eur. J. Biochem.42, 89–95
Heldt, H.W., Chon, C.J., Maronde, D., Herold, A., Stankovic, Z.S., Walker, D.A., Kraminer, A., Kirk, M.R., Heber, U. (1977) Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol.59, 1146–1155
Jones, T.W.A., Pichersky, E., Gottlieb, L.D. (1986) Enzyme activity in EMS-induced null mutations of duplicated genes encoding phosphoglucose isomerase inClarkia. Genetics113, 101–114
Kacser, H., Burns, J.A. (1973) The control of flux. Symp. Soc. Exp. Biol.27, 65–107
Kacser, H., Burns, J.A. (1981) The molecular basis of dominace. Genetics97, 639–666
Kacser, H., Porteous, J. (1987) Control of metabolism: what do we have to measure? Trends Biochem. Sci.12, 5–14
Kruckeberg, A.L., Neuhaus, H.E., Feil, R., Gottlieb, L.D., Stitt, M. (1989) Decreased-activity mutants of phosphoglucose isomerase in the cytosol and chloroplast ofClarkia xantiana. I Impact on mass action ratios and fluxes to sucrose and starch, and estimation of flux control coefficients and elasticity coefficients. Biochem. J.261, 457–467
Leegood, R.C., Labate, C.A., Huber, S.C., Neuhaus, H.E., Stitt, M. (1988) Phosphate sequestration by glycerol and its effects on photosynthetic carbon assimilation by leaves. Planta176, 117–126
Lin, T.-P., Caspar, T., Somerville, C.R., Preiss, J. (1988) A starch deficient mutant ofArabidopsis thaliana with low ADP glucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol.88, 1175–1181
Neuhaus, H.E., Kruckeberg, A.L., Feil, R., Stitt, M. (1989) Reduced-activity mutants of phosphoglucose isomerase in the cytosol and chloroplast ofClarkia xantiana. II. Study of the mechanisms which regulate photosynthate partitioning. Planta178, 110–122
Neuhaus, H.E., Quick, W.P., Siegl, G., Stitt, M. (1990) Control of photosynthate partitioning in spinach leaves: analysis of the interaction between feedforward and feedback regulation of sucrose synthesis. Planta181, 583–592
Newsholme, E.A., Start, C. (1973) Regulation in metabolism. Wiley, Chichester
Preiss, J. (1982) Regulation of the biosynthesis and degradation of starch. Annu. Rev. Plant Physiol.33, 431–454
Preiss, J. (1984) Bacterial glycogen synthesis and its regulation. Annu. Rev. Microbiol.38, 419–458
Preiss, J. (1989) Chemistry and metabolism of internal reserves. In: Bacteria in nature, vol. 3. pp. 189–258. Poindexter, J.S., Leadbetter, E.R., eds., Plenum Press, New York
Preiss, J., Walsh, D.A. (1981) The comparative biochemistry of glycogen and starch. In: Biology of carbohydrates, vol. I, pp. 199–314, Ginsberg, V., ed., Wiley, New York
Quick, W.P., Stitt, M. (1989) An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochim. Biophys. Acta977, 287–296
Quick, W.P., Siegl, G., Neuhaus, H.E., Feil, R., Stitt, M. (1989) Short-term water stress leads to a stimulation of sucrose synthesis by activating sucrose-phosphate synthase. Planta177, 535–546
Rolleston, F.S. (1972) A theoretical background to the use of measured intermediates in the study of the control of intermediary metabolism. Curr. Top. Cell. Regul.5, 47–75
Sharkey, T.D. (1989) Evaluating the role of RUBISCO regulation in photosynthesis of C-3 plants. Philos. Trans. R. Soc. London, Ser. B323, 435–448
Sicher, R.C. (1989) Evidence for a light-dependent increase of phosphoglucomutase activity in isolated intact spinach chloroplasts. Plant Physiol.89, 557–563
Smith, A.M., Neuhaus, H.E., Stitt, M. (1990) The impact of decreased starch-branching enzyme activity on photosynthetic starch synthesis in leaves of wrinkled-seeded peas. Planta181, 310–315
Sowokinos, J.R. (1976) Pyrophosphorylases inSolanum tuberosum. I Changes in ADPglucose and UDPglucose phosphorylase activities associated with starch biosynthesis during tuberisation, maturation and storage of potatoes. Plant Physiol.57, 63–68
Stitt, M. (1989) Control of sucrose synthesis: estimation of free energy charges, the contribution of equilibrium and non-equilibrium reactions, and elasticity and flux control coefficients. In: Techniques and new developments in photosynthesis research, pp. 365–392, Barber, J., Malkin, R., eds. Plenum Press, New York
Stitt, M. (1990) Application of control analysis to photosynthetic sucrose synthesis. In: Control of metabolic processes, pp. 363–376, Cornish-Bowden, A., ed. Plenum Press, New York
Stitt, M., Quick, W.P. (1989) Photosynthetic sucrose synthesis; it's regulation and possibilities for manipulation. Physiol. Plant.77, 633–641
Stitt, M., Gerhardt, R., Wilke, I., Heldt, H.W. (1987) The contribution of fructose-2,6-bisphosphate to the regulation of sucrose synthesis during photosynthesis. Physiol. Plant.69, 377–386
Torres, N.U., Mateo, F., Melendez-Hevia, E., Kacser, H. (1986) Kinetics of metabolic pathways. A system in vivo to study the control of flux. Biochem. J.234, 169–174
Weiner, H., Stitt, M., Heldt, H.W. (1987) Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim. Biophys. Acta893, 13–21
Woodrow, I.E. (1986) Control of the rate of photosynthetic carbon dioxide fixation. Biochim. Biophys. Acta851, 181–192
Woodrow, I.E., Mott, K.A. (1987) Quantitative assessment of the degree to which RUBISCO determines the steady-state rate of photosynthesis during sun-shade acclimation inHelianthus annuus L. Aust. J. Plant Physiol.15, 253–262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ekkehard Neuhaus, H., Stitt, M. Control analysis of photosynthate partitioning. Planta 182, 445–454 (1990). https://doi.org/10.1007/BF02411398
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02411398