Summary
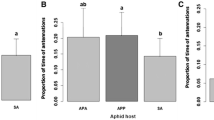
Populations of the milkweed-oleander aphid,Aphis nerii, were sampled in California, Iowa and Puerto Rico. Among these localities the aphid's host plants differ greatly in permanence. I compared populations for migratory potential, measured as the proportion of winged offspring produced in response to being crowded, and for life history and morphometric traits of the subsequent adult winged aphids. I predicted a negative correlation between degree of host plant permanence and migratory potential. As predicted, aphids from Iowa, where migration on to temporary hosts must occur each year, produce a greater proportion of winged offspring (37.7%) than those from California (25.7%) or Puerto Rico (31.6%) where hosts are more permanent. However, hosts in Puerto Rico appear to be more permanent than those in California, yet the difference between populations for migratory potential was opposite to that predicted. Within California the prediction again held: aphids collected from the most impermanent sites produce the greatest proportion of winged offspring. There were no population differences for any life history or morphometric traits of winged aphids that are important contributors to fitness or migratory ability such as time to reproductive maturity, fecundity or wing length. Nor did any traits covary with migratory potential. Thus, there does not appear to be an association of life history and morphology with migratory potential that could enhance the colonizing ability of migrant aphids. I was unable to detect population differentiation for life history and morphology even though there is ample genetic variation within populations on which selection could act and an absence of constraints arising from genetic correlations that could prevent appropriate evolution of traits within populations. The exploitation of temporary host plants therefore occurs by an increase in the number of colonists produced and not by change in life history or morphology of those colonists.
Similar content being viewed by others
References
Blackman, R.L. and Eastop, V.F. (1984)Aphids on the World's Crops: An Identification and Information Guide. Wiley-Interscience, IL, USA.
Bodenheimer, F.S. and Swirski, E. (1957)The Aphidoidea of the Middle East. Weizmann Science Press of Israel, Jerusalem.
Bristow, C.M. Varietal effects on settling and colonization of oleander by alate oleander aphids (Homoptera: Aphididae). Submitted.
Burns, M. (1971) Flight in the vetch aphid,Megoura viciae Buckton. Ph.D. thesis, University of Glasgow, UK.
Clark, A.G. (1987) Genetic correlations: The quantitative genetics of evolutionary constraints.Genetic Constraints on Adaptive Evolution. V. Loeschke (ed.), pp 25–46. Springer-Verlag, New York, USA.
Denno, R.F. (1985) Fitness, population dynamics and migration in planthoppers: The role of host plants.Migration: Mechanisms and Adaptive Significance. M.A. Rankin (ed.), Contrib. Mar. Sci. (Suppl.)27, 623–40.
Dingle, H. (1980) Ecology and evolution of migration.Animal Migration, Orientation, and Navigation. S.A. Gauthreaux, Jr. (ed.), pp. 1–101. Academic Press, New York, USA.
Dingle, H. (1985) Migration and life histories.Migration: Mechanisms and Adaptive Significance. M.A. Rankin (ed.),Contrib. Mar. Sci. (Suppl.)27, 27–42.
Dingle, H. (1986) Evolution and genetics of insect migration.Insect Flight: Dispersal and Migration. W. Danthanarayana (ed.), pp. 11–26. Springer-Verlag, New York, USA.
Dingle, H., Evans, K.E. and Palmer, J.O. (1988) Responses to selection among life-history traits in a nonmigratory population of milkweed bugs (Oncopeltus fasciatus).Evolution 41, 79–92.
Dixon, A.F.G. (1977) Aphid ecology: life cycles, polymorphism and population regulation.Ann. Rev. Ecol. Syst. 8, 329–53.
Dixon, A.F.G. (1984) Plant architectural complexity and alary polymorphism in tree-dwelling aphids.Ecol. Ent. 9, 117–18.
Dixon, A.F.G. (1985)Aphid Ecology. Blackie, Glasgow, UK.
Dixon, A.F.G., Burns, M.D. and Wangboonkong, S. (1968) Migration in aphids: Response to current adversity.Nature 220, 1337–8.
Groeters, F.R. and Dingle, H. The cost of being able to fly in the milkweed-oleander aphid,Aphis nerii (Homomptera: Aphididae).Evol. Ecol. 3, 313–326.
Hall, R.W. and Ehler, L.E. (1980) Population ecology ofAphis nerii on oleander.Envir. Ent. 9, 338–44.
Hamilton, W.D. and May, R.M. (1977) Dispersal in stable habitats.Nature 269, 578–81.
Harrison, R.G. (1980) Dispersal polymorphisms in insects.Ann. Rev. Ecol. Syst. 11, 95–118.
Janzen, D.H. (1977) What are dandelions and aphids?Amer. Natur. 111, 586–9.
Kvenberg, J.E. and Jones, P.A. (1974) Comparison of alate offspring produced by two biotypes of the greenbug.Envr. Ent. 3, 407–8.
Lamb, R.J. and MacKay, P.A. (1979) Variability in migratory tendency within and among natural populations of the pea aphid,Acyrthosiphon pisum.Oecologia 39, 289–99.
Lenski, R.E. and Service, P.M. (1982) The statistical analysis of population growth rates calculated from schedules of survivorship and fecundity.Ecology 63, 655–62.
Palmer, J.O. (1985) Ecological genetics of wing length, flight propensity, and early fecundity in a migratory insect.Migration: Mechanisms and Adaptive Significance. M.A. Rankin (ed.),Contrib. Mar. Sci. (Suppl.)27, 663–73.
Roff, D.A. (1977) Dispersal in Dipterans: Its costs and consequences.J. Anim. Ecol. 46, 443–56.
Roff, D.A. (1984) The cost of being able to fly: A study of wing polymorphism in two species of crickets.Oecologia 63, 30–7.
SAS Institute (1985)SAS User's Gudie: Statistics. SAS Institute, Cary, NC, USA.
Solbreck, C. (1978) Migration, diapause and direct development as alternative life histories in a seed bug,Neacoryphus bicrucis. Evolution of Insect Migration and Diapause. H. Dingle (ed.), pp. 195–217. Springer-Verlag, New York, USA.
Southwood, T.R.E. (1962) Migration of terrestrial arthropods in relation to habitat.Biol. Rev. 37, 171–214.
Southwood, T.R.E. (1977) Habitat, the templet for ecological strategies?J. Anim. Ecol. 46, 337–65.
Southwood, T.R.E. (1987) Habitat and insect biology.Bull. Ent. Soc. Amer. 33, 211–14.
Taylor, L.R. and Taylor, R.A.J. (1977) Aggregation, migration and population mechanics.Nature 265, 415–21.
Vepsalainen, K. (1978) Wing dimorphism and diapause inGerris: Determination and adaptive significance.Evolution of Insect Migration and Diapause. H. Dingle (ed.), pp 218–53. Springer-Verlag, New York, USA.
Waloff, N. (1983) Absence of wing polymorphism in the arboreal, phytophagous species of some taxa of temperate Hemiptera: An hypothesis.Ecol. Ent. 8, 228–32.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Groeters, F.R. Geographic and clonal variation in the milkweed-oleander aphid,Aphis nerii (Homoptera: Aphididae), for winged morph production, life history, and morphology in relation to host plant permanence. Evol Ecol 3, 327–341 (1989). https://doi.org/10.1007/BF02285263
Issue Date:
DOI: https://doi.org/10.1007/BF02285263