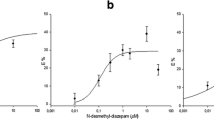
Abstract
Clozapine, an atypical neuroleptic, functionally antagonizes the γ-aminobutyric acid-induced chloride uptake via the main central inhibitory receptor, γ-aminobutyric acid type A (GABAA) receptor, in brain vesicles. GABAA antagonism by micromolar concentrations of clozapine is more efficient in rat cerebrocortical and hippocampal membranes than in cerebellar membranes, as evidenced by clozapine reversal GABA-inhibition of [35S]t-butylbicyclophosphorothionate ([35S]TBPS) binding. A typical neuroleptic, haloperidol, failed to antagonize GABA in any of these brain regions, while the specific GABAA antagonist 2′-(3′-carboxy-2′,3′-propyl)-3-amino-6-p-methoxyphenylpyrazinium bromide (SR 95531) was efficient in all three brain regions. Clozapine action on [35S]TBPS binding was unaffected by the benzodiazepine receptor antagonist flumazenil. Clozapine inhibited the binding of [3H]muscimol and [3H]SR 95531 to the GABA recognition site, but this effect only partially correlated with the regional differences in and the potency of clozapine antagonism of GABA-inhibition of [35S]TBPS binding, suggesting that also other than GABA sites may mediate clozapine actions. Autoradiography of [35S]TBPS binding revealed GABA antagonism by clozapine in most brain regions. Main exceptions were cerebellar granule cell and molecular layers, olfactory bulb external plexiform and glomerular layers and primary olfactory cortex, where clozapine antagonized GABA inhibition less than average, and lateral hypothalamic and preoptic areas where its antagonism was greater than average. Recombinant α6β2γ2 receptors, the predominant α6 subunit-containing receptor subtype in cerebellar granule cells, failed to show GABA antagonism by clozapine up to 100 μM. In contrast, recombinant α1β2γ2 receptors, forming the predominant receptor subtype in the brain, were clozapine sensitive. Recombinant α6β2γ2 and α6β3γ2 receptors resulted in clozapine-insensitive receptors, whereas α6β1γ2 receptors were clozapine sensitive. The efficacy of clozapine to antagonize GABA in α1βxγ2 receptors decreased in the order of α1β1γ2>α1β2γ2>α1β3γ2. The results indicate that clozapine antagonizes the function of most GABAA receptor subtypes, and that the interaction is determined by the interaction of the α and β subunit variants. GABA antagonism is a unique property of clozapine, not shared by haloperidol, which might be involved in the pharmacological mechanism for the increased seizure susceptibility associated with clozapine treatment.
Similar content being viewed by others
References
Angst J, Jaenicke U, Padrutt A, Scharfetter C (1971) Ergebnisse eines Doppelblindversuches von Clozapin (8-chlo-11-(4-methyl-1-piperazinyl)-5H-dibenzo-(b,e)-1,4)-diazepin) im Vergleich zu Levomepromazin. Pharmacopsychiatry 4: 192–200
Baldessarini RJ, Frankenburg FR (1991) Clozapine — a novel antipsychotic agent. N Engl J Med 324:746–754
Benke D, Fritschy J-M, Trzeciak A, Bannwarth W, Mohler H (1994) Distribution, prevalence, and drug binding profile of γ-aminobutyric acid type A receptor subtypes differing in the β-subunit variant. J Biol Chem 269:27100–27107
Chen C, Okayama H (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745–2752
Choc MG, Lehr RG, Hsuan F, Honigfeld G, Smith HT, Borison R, Volavka J (1987) Multiple-dose pharmacokinetics of clozapine in patients. Pharm Res 4:402–405
Conn PJ, Sanders-Bush E (1985) Serotonin-stimulated phosphoinositide turnover: mediation by the S2 binding site in rat cerebral cortex but not in subcortical regions. J Pharmacol Exp Ther 234:195–203
Coward DM (1992) General pharmacology of clozapine. Br J Psychiatry 160 [Suppl 17]:5–11
Deutch AY, Moghaddam B, Innis RB, Krystal JH, Aghajanian GK, Bunney BS, Chamey DS (1991) Mechanisms of action of atypical antipsychotic drugs. Implications for novel therapeutic strategies for schizophrenia. Schizophr Res 4:121–156
Devinsky O, Honigfeld G, Patin J (1991) Clozapine-related seizures. Neurology 41:369–371
Edgar PP, Schwartz RD (1990) Localization and characterization of 35S-t-butylbicyclophosphorothionate binding in rat brain: an autoradiographic study. J Neurosci 10:603–612
Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544
Gaertner HJ, Fischer E, Hoss J (1989) Side effects of clozapine. Psychopharmacology 99:S97-S100
Haring C, Neudorfer C, Schwitzer J, Hummer M, Saria A, Hinterhuher H, Fleischhacker WW (1994) EEG alterations in patients treated with clozapine in relation to plasma levels. Psychopharmacology 114:97–100
Hartvig P, Eckemäs S, Lindström L, Ekblom B, Bondesson U, Lundqvist H, Halldin C, Någren K, Långström B (1986) Receptor binding of N-(methyl-11C)clozapine in the brain of rhesus monkey studied by positron emission tomography (PET). Psychopharmacology 89:248–252
Heaulme M, Chambon JP, Leyris R, Molimard JC, Wermuth CG, Biziere K (1986) Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site. Brain Res 384:224–231
Hunkeler W, Möhler H, Pieri L, Pole P, Bonetti EP, Cumin R, Schaffner R, Haefely W (1981) Selective antagonists of benzodiazepines. Nature 290:514–516
Idänpää-Heikkilä J, Alhava E, Olkinuora M, Palva IP (1977) Agranulocytosis during treatment with clozapine. Eur J Clin Pharmacol 11:193–198
Im WB, Blakeman DP (1991) Correlation between γ-aminobutyric acidA receptor ligand-induced changes in t-butylbicyclopho-sphoro[35S]thionate binding and 36Cl− uptake in rat cerebrocortical membranes. Mol Pharmacol 39:394–398
Kane JM (1992) Clinical efficacy of clozapine in treatment-refractory schizophrenia: an overview. Br J Psychiatry 160 [Suppl 17]:41–45
Kane JM, Honigfeld G, Singer J, Meltzer H (1988) Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 45:789–796
Korpi ER, Lüddens H (1993) Regional γ-aminobutyric acid sensitivity of t-butylbicyclophosphoro[35S]thionate binding depends on γ-aminobutyric acidA receptor a subunit. Mol Pharmacol 44:87–92
Korpi ER, Uusi-Oukari M (1989) GABAA receptor-mediated chloride flux in brain homogenates from rat lines with differing innate alcohol sensitivities. Neuroscience 32:387–392
Korpi ER, Kleinman JE, Costakos DT, Linnoila M, Wyatt RJ (1984) Reduced haloperidol in the post-mortem brains of haloperidol-treated patients. Psychiatry Res 11:259–269
Korpi ER, Lüddens H, Seeburg PH, (1992) GABAA antagonists reveal binding sites for [35S]TBPS in cerebellar granular cell layer. Eur J Pharmacol 211:427–428
Korpi ER, Kleingoor C, Kettenmann H, Seeburg PH (1993) Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature 361:356–359
Korpi ER, Kuner T, Seeburg PH, Lüddens H (1995) Selective antagonist for the cerebellar granule cell-specific γ-aminobutyric acid type A receptor. Mol Pharmacol 47:283–289
Kuoppamäki M, Syvälahti E, Hietala J (1993) Clozapine and N-desmethylelozapine are potent 5-HT1C receptor antagonists. Eur J Pharmacol — Mol Pharmacol Sect 245:179–182
Laurie DJ, Seeburg PH, Wisden W (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci 12:1063–1076
Lloyd KG, Dreksler S (1979) An analysis of [3H]gamma-aminobutyric acid (GABA) binding in the human brain. Brain Res 163:77–87
Lüddens H, Korpi ER (1995) Biological function of GABAA/benzodiazepine receptor heterogeneity. J Psychiatry Res 29:77–94
Lüddens H, Wisden W (1991) Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci 12:49–51
Lüddens H, Pritchett DB, Kohler M, Mllisch I, Keinänen K, Monyer H, Sprengel R, Seeburg PH (1990) Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature 346:648–651
Lüddens H, Seeburg PH, Korpi ER (1994) Impact of β and γ variants on ligand-binding properties of γ-aminobutyric acid type A receptors. Mol Pharmacol 45:810–814
Maksay G, Simonyi M (1986) Kinetic regulation of convulsant (TBPS) binding by GABAergic agents. Mol Pharmacol 30:321–328
Maksay G, Simonyi M (1988) Nonequilibrium modulation of 35S-TBPS binding by benzodiazepine agonists and antagonists. Biochem Pharmacol 37:2195–2200
Messing RO, Closson RG, Simon RP (1984) Drug-induced seizures: a 10-year experience. Neurology 34:1582–1586
Olsen RW, Tobin AJ (1990) Molecular biology of GABAA receptors. FASEB J 4:1469–1480
Olsen RW, McCabe RT, Wamsley JK (1990) GABAA receptor subtypes: autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J Chem Neuroanat 3:59–76
Pacia SV, Devinsky O (1994) Clozapine-related seizures: experience with 5,629 patients. Neurology 44:2247–2249
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, New York
Peroutka SJ, Snyder SH (1980) Relationship of neuroleptic drug effects at brain dopamine, serotonin, α-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry 137:1518–1522
Persohn E, Malherbe P, Richards JG (1992) Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol 326:193–216
Pritchett DB, Lüddens H, Seeburg PH (1989a) Type I and Type II GABAA-benzodiazepine receptors produced in transfected cells. Science 245:1389–1392
Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH (1989b) Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 338:582–585
Schotte A, Janssen PFM, Megens AAHP, Leysen JE (1993) Occupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiography. Brain Res 631:191–202
Seeman P (1992) Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology 7:261–284
Seeman P, Van Tol HHM (1994) Dopamine receptor pharmacology. Trends Pharmacol Sci 15:264–270
Squires RF, Saederup E (1991) A review of evidence for GABAergic predominance/glutamatergic deficit as a common etiological factor in both schizophrenia and affective psychoses: more support for a continuum hypothesis of “functional” psychosis. Neurochem Res 16:1099–1111
Squires RF, Saederup E (1993) Mono N-aryl ethylenediamine and piperazine derivatives are GABAA receptor blockers: implications for psychiatry. Neurochem Res 18:787–793
Squires RF, Casida JE, Richardson M., Saederup E (1983) [35S]t-Bu-tylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to γ-aminobutyric acid-A and ion recognition sites. Mol Pharmacol 23:326–336
Van Tol HHM, Bungow JR, Guan H-C, Sunahara RK, Seeman P, Niznik HB, Civelli O (1991) Cloning of a human dopamine D4 receptor gene with high affinity for the antipsychotic clozapine. Nature 350:614–619
Wisden W, Seeburg PH (1992) GABAA receptor channels: from subunits to functional entities. Curt Opin Neurobiol 2:263–269
Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040–1062
Wood PL, Loo P, Braunwalder A, Yokoyama N, Cheney DL (1984) In vitro characterization of benzodiazepine receptor agonists, antagonists, inverse agonists and agonist/antagonists. J Pharmacol Exp Ther 231:572–576
Ymer S, Schofield PR, Draguhn A, Werner P, Köhler M, Seeburg PH (1989) GABAA receptor β subunit heterogeneity: functional expression of cloned cDNAs. EMBO J 8:1665–1670
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korpi, E.R., Wong, G. & Lüddens, H. Subtype specificity of γ-aminobutyric acid type A receptor antagonism by clozapine. Naunyn-Schmiedeberg's Arch Pharmacol 352, 365–373 (1995). https://doi.org/10.1007/BF00172773
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00172773