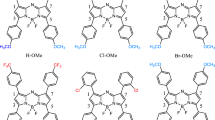
Abstract
During the last three decades, the boradipyrromethene (BODIPY) dyes are established as one of the most versatile class of organic dyes for various optical applications in almost every field of science. Unique photophysical properties and rich redox behaviours are the most attracting features of these dyes. Along with these, their synthetic versatility made them attractive to scientific fraternity. Different synthetic strategies were adopted to tune their optical properties to synthesize custom made dyes for various applications. In this chapter, molecular designing of BODIPY dyes for tuning their optical properties are discussed. Finally, the applications of the BODIPY dyes as laser dyes, chemical/bio-sensors, cellular imaging agents, triplet photosensitizers in photodynamic therapy, fluorescent-Positron Emission Tomography (PET) probes, solar energy conversion agents in organic photovoltaics (OPV) and, in photocatalysis and developing self-assembled architectures are discussed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Atilgan, S., Ekmekci, Z., Dogan, A.L., Guc, D., Akkaya, E.U.: Chem. Commun. 4398–4400 (2006)
Bartelmess, J., Francis, A.J., El Roz, K.A., Castellano, F.N., Weare, W.W., Sommer, R.D.: Inorg. Chem. 53, 4527–4534 (2014)
Belmonte-Vázquez, J.L., Avellanal-Zaballa, E., Enríquez-Palacios, E., Cerdán, L., Esnal, I., Bañuelos, J., Villegas-Gómez, C., Arbeloa, I.L., Peña-Cabrera, E.: J. Org. Chem. 84, 2523–2541 (2019)
Bessette, A., Hanan, G.S.: Chem. Soc. Rev. 43, 3342–3405 (2014)
Bodio, E., Goze, C.: Dyes Pigm. 160, 700–710 (2019)
Boens, N., Leen, V., Dehaen, W.: Chem. Soc. Rev. 41, 1130–1172 (2012)
Boyer, J.H., Haag, A.M., Sathyamoorthi, G., Soong, M.-L., Thangaraj, K., Pavlopoulos, T.G.: Heteroat. Chem. 4, 39–49 (1993)
Brown, S.B., Brown, E.A., Walker, I.: Lancet Oncol. 5, 497–508 (2004)
Bulut, I., Huaulmé, Q., Mirloup, A., Chávez, P., Fall, S., Hébraud, A., Méry, S., Heinrich, B., Heiser, T., Lévêque, P., Leclerc, N.: Chemsuschem 10, 1878–1882 (2017)
Buyukcakir, O., Bozdemir, O.A., Kolemen, S., Erbas, S., Akkaya, E.U.: Org. Lett. 11, 4644–4647 (2009)
Cakmak, Y., Kolemen, S., Duman, S., Dede, Y., Dolen, Y., Kilic, B., Kostereli, Z., Yildirim, L.T., Dogan, A.L., Guc, D., Akkaya, E.U.: Angew. Chem. Int. Ed. 50, 11937–11941 (2011)
Carlucci, G., Carney, B., Brand, C., Kossatz, S., Irwin, C.P., Carlin, S.D., Keliher, E.J., Weber, W., Reiner, T.: Mol. Imaging Biol. 17, 848–855 (2015)
Dodani, S.C., He, Q., Chang, C.J.: J. Am. Chem. Soc. 131, 18020–18021 (2009)
Dura, L., Ahrens, J., Pohl, M.-M., Höfler, S., Bröring, M., Beweries, T.: Chem. Eur. J. 21, 13549–13552 (2015)
Er, J.C., Tang, M.K., Chia, C.G., Liew, H., Vendrell, M., Chang, Y.-T.: Chem. Sci. 4, 2168–2176 (2013)
Ethirajan, M., Chen, Y., Joshi, P., Pandey, R.K.: Chem. Soc. Rev. 40, 340–362 (2011)
Gabe, Y., Urano, Y., Kikuchi, K., Kojima, H., Nagano, T.: J. Am. Chem. Soc. 126, 3357–3367 (2004)
Gorai, S., Ghosh, A., Chakraborty, S., Retailleau, P., Ghanty, T.K., Patro, B.S., Mula, S.: Dyes Pigm. 203, 110343 (2022)
Gorman, A., Killoran, J., O’Shea, C., Kenna, T., Gallagher, W.M., O’Shea, D.F.: J. Am. Chem. Soc. 126, 10619–10631 (2004)
Gupta, M., Mula, S., Tyagi, M., Ghanty, T.K., Murudkar, S., Ray, A.K., Chattopadhyay, S.: Chem. Eur. J. 19, 17766–17772 (2013)
Gupta, M., Mula, S., Ghanty, T.K., Naik, D.B., Ray, A.K., Sharma, A., Chattopadhyay, S.: J. Photochem. Photobiol Chem. 349, 162–170 (2017)
Harriman, A., Mallon, L.J., Elliot, K.J., Haefele, A., Ulrich, G., Ziessel, R.: J. Am. Chem. Soc. 131, 13375–13386 (2009)
Hendricks, J.A., Keliher, E.J., Wan, D., Hilderbrand, S.A., Weissleder, R., Mazitschek, R.: Angew. Chem. Int. Ed. 51, 4603–4606 (2012)
Huang, L., Zhao, J., Guo, S., Zhang, C., Ma, J.: J. Org. Chem. 78, 5627–5637 (2013)
Jagtap, K.K., Shivran, N., Mula, S., Naik, D.B., Sarkar, S.K., Mukherjee, T., Maity, D.K., Ray, A.K.: Chem. Eur. J. 19, 702–708 (2013)
Jia, M.-Y., Wang, Y., Liu, Y., Niu, L.-Y., Feng, L.: Biosens. Bioelectron. 85, 515–521 (2016)
Jiang, N., Fan, J., Liu, T., Cao, J., Qiao, B., Wang, J., Gao, P., Peng, X.: Chem. Commun. 49, 10620–10622 (2013)
Jiang, X.-D., Zhao, J., Li, Q., Sun, C.-L., Guan, J., Sun, G.-T., Xiao, L.-J.: Dyes Pigm. 125, 136–141 (2016)
Jiao, L., Yu, C., Uppal, T., Liu, M., Li, Y., Zhou, Y., Hao, E., Hub, X., Vicente, M.G.H.: Org. Biomol. Chem. 8, 2517–2519 (2010)
Kamkaew, A., Lim, S.H., Lee, H.B., Kiew, L.V., Chung, L.Y., Burgess, K.: Chem. Soc. Rev. 42, 77–88 (2013)
Killoran, J., Allen, L., Gallagher, J.F., Gallagher, W.M., O′Shea, D.F.: Chem. Commun. 1862–1863 (2002)
Kim, H., Kim, K., Son, S.-H., Choi, J.Y., Lee, K.-H., Kim, B.-T., Byun, Y., Choe, Y.S.: ACS Chem. Neurosci. 10, 1445–1451 (2019)
Kollmannsberger, M., Rurack, K., Resch-Genger, U., Daub, J.: J. Phys. Chem. A 102, 10211–10220 (1998)
Li, W., Li, L., Xiao, H., Qi, R., Huang, Y., Xie, Z., Jing, X., Zhang, H.: RSC Adv. 3, 13417–13421 (2013)
Loudet, A., Burgess, K.: Chem. Rev. 107, 4891–4932 (2007)
Lu, H., Mack, J., Yang, Y., Shen, Z.: Chem. Soc. Rev. 43, 4778–4823 (2014)
Lundrigan, T., Crawford, S.M., Cameron, T.S., Thompson, A.: Chem. Commun. 48, 1003–1005 (2012)
Maity, A., Sarkar, A., Sil, A., BN, S.B., Patra, S.K.: New J. Chem. 41, 2296–2308 (2017)
Mbatia, H.W., Kennedy, D.P., Camire, C.E., Incarvito, C.D., Burdette, S.C.: Eur. J. Inorg. Chem. 2010, 5069–5078 (2010)
Mora, A.K., Murudkar, S., Shivran, N., Mula, S., Chattopadhyay, S., Nath, S.: Int. J. Biol. Macromol. 166, 1121–1130 (2021)
More, A.B., Mula, S., Thakare, S., Sekar, N., Ray, A.K., Chattopadhyay, S.: J. Org. Chem. 79, 10981–10987 (2014)
More, A.B., Mula, S., Thakare, S., Chakraborty, S., Ray, A.K., Sekar, N., Chattopadhyay, S.: J. Lumin. 190, 476–484 (2017)
Mula, S., Ray, A.K., Banerjee, M., Chaudhuri, T., Dasgupta, K., Chattopadhyay, S.: J. Org. Chem. 73, 2146–2154 (2008)
Mula, S., Ulrich, G., Ziessel, R.: Tetrahedron Lett. 50, 6383–6388 (2009)
Mula, S., Elliott, K., Harriman, A., Ziessel, R.: J. Phys. Chem. A 114, 10515–10522 (2010)
Mula, S., Frein, S., Russo, V., Ulrich, G., Ziessel, R., Barberá, J., Deschenaux, R.: Chem. Mater. 27, 2332–2342 (2015)
Nguyen, Q.T., Olson, E.S., Aguilera, T.A., Jiang, T., Scadeng, M., Ellies, L.G., Tsien, R.Y.: Proc. Natl. Acad. Sci. U S A 107, 4317–4322 (2010)
Olivier, J.-H., Barberá, J., Bahaidarah, E., Harriman, A., Ziessel, R.: J. Am. Chem. Soc. 134, 6100–6103 (2012)
Ortiz, M.J., Agarrabeitia, A.R., Duran-Sampedro, G., Bañuelos Prieto, J., Lopez, T.A., Massad, W.A., Montejano, H.A., García, N.A., Arbeloa, I.L.: Tetrahedron 68, 1153–1162 (2012)
Quan, L., Gu, J., Lin, W., Wei, Y., Lin, Y., Liu, L., Ding, H., Pan, C., Xie, Z., Wu, T.: Chem. Commun. 55, 8564–8566 (2019)
Ray, A.K., Kundu, S., Sasikumar, S., Rao, C.S., Mula, S., Sinha, S., Dasgupta, K.: Appl. Phys. B 87, 483–488 (2007)
Ray, C., Schad, C., Moreno, F., Maroto, B.L., Bañuelos, J., Arbeloa, T., García-Moreno, I., Villafuerte, C., Muller, G., de la Moya, S.: J. Org. Chem. 85, 4594–4601 (2020)
Sen, A., Mora, A.K., Koli, M., Mula, S., Kundu, S., Nath, S.: Int. J. Biol. Macromol. 220, 901–909 (2022)
Shah, M., Thangaraj, K., Soong, M.-L., Wolford, L.T., Boyer, J.H., Politzer, I.R., Pavlopoulos, T.G.: Heteroat. Chem. 1, 389–399 (1990)
Shivran, N., Mula, S., Ghanty, T.K., Chattopadhyay, S.: Org. Lett. 13, 5870–5873 (2011)
Shivran, N., Tyagi, M., Mula, S., Gupta, P., Saha, B., Patro, B.S., Chattopadhyay, S.: Eur. J. Med. Chem. 122, 352–365 (2016)
Shivran, N., Koli, M.R., Chakraborty, G., Srivastava, A.P., Chattopadhyay, S., Mula, S.: Org. Biomol. Chem. 19, 7920–7929 (2021)
Srinivasa Rao, R., Bagui, A., Hanumantha Rao, G., Gupta, V., Singh, S.P.: Chem. Commun. 53, 6953–6956 (2017)
Treibs, A., Kreuzer, F.-H.: Justus Liebigs Ann. Chem. 718, 208–223 (1968)
Tsien, R.Y.: Nat. Rev. Mol. Cell Biol. Suppl. Ss16–21 (2003)
Turksoy, A., Yildiz, D., Akkaya, E.U.: Coord. Chem. Rev. 379, 47–64 (2019)
Ulrich, G., Ziessel, R., Harriman, A.: Angew. Chem. Int. Ed. 47, 1184–1201 (2008)
Valeur, B., Berberan-Santos, M.N.: Molecular Fluorescence: Principles and Applications, 2nd edn. Wiley-VCH Verlag GmbH & Co, KGaA (2012)
Wagner, R.W., Lindsey, J.S.: Pure Appl. Chem. 68, 1373–1380 (1996)
Wainwright, M.: Chem. Soc. Rev. 25, 351–359 (1996)
Wang, L., Xiao, Y., Tian, W., Deng, L.: J. Am. Chem. Soc. 135, 2903–2906 (2013)
Wang, J.-L., Zhang, L., Gao, L.-X., Chen, J.-L., Zhou, T., Liu, Y., Jiang, F.-L.: J Mater Chem B 9, 8639–8645 (2021)
Yang, Z., He, Y., Lee, J.-H., Park, N., Suh, M., Chae, W.-S., Cao, J., Peng, X., Jung, H., Kang, C., Kim, J.S.: J. Am. Chem. Soc. 135, 9181–9185 (2013)
Yogo, T., Urano, Y., Ishitsuka, Y., Maniwa, F., Nagano, T.: J. Am. Chem. Soc. 127, 12162–12163 (2005)
Zhang, X., Yu, H., Xiao, Y.: J. Org. Chem. 77, 669–673 (2012)
Zhang, S., Wu, T., Fan, J., Li, Z., Jiang, N., Wang, J., Dou, B., Sun, S., Song, F., Peng, X.: Org. Biomol. Chem. 11, 555–558 (2013)
Zhang, H.-X., Chen, J.-B., Guo, X.-F., Wang, H., Zhang, H.-S.: Anal. Chem. 86, 3115–3123 (2014)
Zhang, D., Martín, V., García-Moreno, I., Costela, A., Pérez-Ojeda, M.E., Xiao, Y.: Chem. Chem. Phys. 13, 13026–13033 (2011)
Zheng, B., Sabatini, R.P., Fu, W.F., Eum, M.S., Brennessel, W.W., Wang, L., McCamant, D.W., Eisenberg, R.: Proc. Natl. Acad. Sci. U S A 112, E3987–E3996 (2015)
Ziessel, R., Ulrich, G., Harriman, A., Alamiry, M.A.H., Stewart, B., Retailleau, P.: Chem. Eur. J. 15, 1359–1369 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mula, S. (2024). BODIPY: A Unique Dye for Versatile Optical Applications. In: Ningthoujam, R.S., Tyagi, A.K. (eds) Handbook of Materials Science, Volume 1. Indian Institute of Metals Series. Springer, Singapore. https://doi.org/10.1007/978-981-99-7145-9_13
Download citation
DOI: https://doi.org/10.1007/978-981-99-7145-9_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-7144-2
Online ISBN: 978-981-99-7145-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)