Abstract
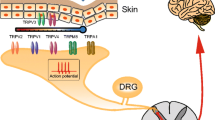
Transient receptor potential vanilloid type 1 (TRPV1) is a nonselective cation channel that is intensively expressed in the peripheral nerve system and involved in a variety of physiological and pathophysiological processes in mammals. Its activity is of great significance in transmitting pain or itch signals from peripheral sensory neurons to the central nervous system. The alteration or hypersensitivity of TRPV1 channel is well evidenced under various pathological conditions. Moreover, accumulative studies have revealed that TRPV1-expressing (TRPV1+) sensory neurons mediate the neuroimmune crosstalk by releasing neuropeptides to innervated tissues as well as immune cells. In the central projection, TRPV1+ terminals synapse with the secondary neurons for the transmission of pain and itch signalling. The intense involvement of TRPV1 and TRPV1+ neurons in pain and itch makes it a potential pharmaceutical target. Over decades, the basis of TRPV1 channel structure, the nature of its activity, and its modulation in pathological processes have been broadly studied and well documented. Herein, we highlight the role of TRPV1 and its associated neurons in sensing pain and itch. The fundamental understandings of TRPV1-involved nociception, pruriception, neurogenic inflammation, and cell-specific modulation will help bring out more effective strategies of TRPV1 modulation in treating pain- and itch-related diseases.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Liao M, Cao E, Julius D, Cheng Y (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–112
Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A (2009) TRPV1 is activated by both acidic and basic pH. J Neurosci 29:153–158
Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493:596–606
Kuruvilla M, Kalangara J, Lee FEE (2019) Neuropathic pain and itch mechanisms underlying allergic conjunctivitis. J Investig Allergol Clin Immunol 29:349–356
Jara-Oseguera A, Simon SA, Rosenbaum T (2008) TRPV1: on the road to pain relief. Curr Mol Pharmacol 1:255–269
McKemy DD (2011) A spicy family tree: TRPV1 and its thermoceptive and nociceptive lineage. EMBO J 30:453–455
Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA (2011) TRPV1-lineage neurons are required for thermal sensation. EMBO J 30:582–593
Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X (2014) Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81:873–887
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K (2020) The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161:1976–1982
Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M (2006) The neurobiology of itch. Nat Rev Neurosci 7:535–547
Saloman JL, Chung MK, Ro JY (2013) P2X(3) and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience 232:226–238
Barabas ME, Stucky CL (2013) TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Mol Pain 9:9
Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, Wang SS, Sun MM, Lu YJ, Zhong YQ, Hu XY, Hou R, Zhou BB, Bao L, Xiao HS, Zhang X (2016) Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 26:967
Li C, Wang S, Chen Y, Zhang X (2018) Somatosensory neuron typing with high-coverage single-cell RNA sequencing and functional analysis. Neurosci Bull 34:200–207
Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P (2015) Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18:145–153
Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S (2018) Molecular architecture of the mouse nervous system. Cell 174:999–1014.e22
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ (2015) A classification of chronic pain for ICD-11. Pain 156:1003–1007
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ (2019) Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160:19–27
Nugraha B, Gutenbrunner C, Barke A, Karst M, Schiller J, Schafer P, Falter S, Korwisi B, Rief W, Treede RD (2019) Pain ITftCoC: the IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain 160:88–94
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284
Jordt SE, Tominaga M, Julius D (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A 97:8134–8139
Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D (2006) Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 444:208–212
Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D (2010) A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141:834–845
Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24:487–517
Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN (2005) Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain 115:296–307
Chen SR, Pan HL (2006) Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol 95:3086–3096
Tang HB, Inoue A, Oshita K, Nakata Y (2004) Sensitization of vanilloid receptor 1 induced by bradykinin via the activation of second messenger signaling cascades in rat primary afferent neurons. Eur J Pharmacol 498:37–43
Abdelhamid RE, Sluka KA (2015) ASICs mediate pain and inflammation in musculoskeletal diseases. Physiology (Bethesda) 30:449–459
Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282
Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 102:12248–12252
Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545
Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 104:13519–13524
Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ (2008) Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 586:3447–3459
Bautista DM, Pellegrino M, Tsunozaki M (2013) TRPA1: a gatekeeper for inflammation. Annu Rev Physiol 75:181–200
Taylor-Clark TE, Undem BJ, Macglashan DW Jr, Ghatta S, Carr MJ, McAlexander MA (2008) Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 73:274–281
McMahon SB, Wood JN (2006) Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell 124:1123–1125
Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM (2007) Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol 583:175–193
Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X (2015) Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron 85:833–846
Moon EH, Kim MJ, Ko KS, Kim YS, Seo J, Oh SP, Lee YJ (2010) Generation of mice with a conditional and reporter allele for Tmem100. Genesis 48:673–678
Weyer AD, Stucky CL (2015) Loosening pain’s grip by tightening TRPV1-TRPA1 interactions. Neuron 85:661–663
Sun YG, Chen ZF (2007) A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448:700–703
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X (2009) Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139:1353–1365
Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ (2001) A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106:619–632
Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X (2013) A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16:174–182
Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X (2012) Mechanisms of itch evoked by β-alanine. J Neurosci 32:14532–14537
Mishra SK, Hoon MA (2010) Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci 43:157–163
Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon SR, Carstens E, Homey B, Basbaum A, Steinhoff M (2014) A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 133:448–460
Meng J, Moriyama M, Feld M, Buddenkotte J, Buhl T, Szollosi A, Zhang J, Miller P, Ghetti A, Fischer M, Reeh PW, Shan C, Wang J, Steinhoff M (2018) New mechanism underlying IL-31-induced atopic dermatitis. J Allergy Clin Immunol 141:1677–1689.e8
Wang F, Trier AM, Li F, Kim S, Chen Z, Chai JN, Mack MR, Morrison SA, Hamilton JD, Baek J, Yang TB, Ver Heul AM, Xu AZ, Xie Z, Dong X, Kubo M, Hu H, Hsieh CS, Dong X, Liu Q, Margolis DJ, Ardeleanu M, Miller MJ, Kim BS (2021) A basophil-neuronal axis promotes itch. Cell 184:422–440.e17
Dimitriadou V, Rouleau A, Dam Trung Tuong M, Newlands GJ, Miller HR, Luffau G, Schwartz JC, Garbarg M (1994) Functional relationship between mast cells and C-sensitive nerve fibres evidenced by histamine H3-receptor modulation in rat lung and spleen. Clin Sci (Lond) 87:151–163
Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U (2007) TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 27:2331–2337
Mishra SK, Hoon MA (2013) The cells and circuitry for itch responses in mice. Science 340:968–971
Campion M, Smith L, Gatault S, Metais C, Buddenkotte J, Steinhoff M (2019) Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp Dermatol 28:1501–1504
Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, Luo J, Gao X, Yang L, Hamilton SL, Wang PL, Brestoff JR, Council ML, Brasington R, Schaffer A, Brombacher F, Hsieh CS, Gereau RW, Miller MJ, Chen ZF, Hu H, Davidson S, Liu Q, Kim BS (2017) Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 171:217–228.e13
Stanger R, Rivera-Oyola R, Lebwohl M (2020) Dupilumab as a treatment for generalized idiopathic pruritus: a report of two cases. Br J Dermatol 182:1494–1495
Zhai LL, Savage KT, Qiu CC, Jin A, Valdes-Rodriguez R, Mollanazar NK (2019) Chronic pruritus responding to dupilumab-a case series. Medicines (Basel) 6(3):72
Calugareanu A, Jachiet M, Tauber M, Nosbaum A, Aubin F, Misery L, Droitcourt C, Barbarot S, Debarbieux S, Saussine A, Bagot M, de Masson A, Seneschal J, Staumont-Salle D, Bouaziz JD, French Group of Research and Study in Atopic Dermatitis from the French Society of Dermatology (2020) Effectiveness and safety of dupilumab for the treatment of prurigo nodularis in a French multicenter adult cohort of 16 patients. J Eur Acad Dermatol Venereol 34:e74–e76
Giura MT, Viola R, Fierro MT, Ribero S, Ortoncelli M (2020) Efficacy of dupilumab in prurigo nodularis in elderly patient. Dermatol Ther 33:e13201
Napolitano M, Fabbrocini G, Scalvenzi M, Nistico SP, Dastoli S, Patruno C (2020) Effectiveness of dupilumab for the treatment of generalized prurigo nodularis phenotype of adult atopic dermatitis. Dermatitis 31:81–84
Tanis R, Ferenczi K, Payette M (2019) Dupilumab treatment for prurigo nodularis and pruritis. J Drugs Dermatol 18:940–942
Jeon J, Wang F, Badic A, Kim BS (2021) Treatment of patients with chronic pruritus of unknown origin with dupilumab. J Dermatolog Treat:1–4
Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK (2009) TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A 106:11330–11335
Kim BM, Lee SH, Shim WS, Oh U (2004) Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett 361:159–162
Han SK, Mancino V, Simon MI (2006) Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron 52:691–703
Jian T, Yang N, Yang Y, Zhu C, Yuan X, Yu G, Wang C, Wang Z, Shi H, Tang M, He Q, Lan L, Wu G, Tang Z (2016) TRPV1 and PLC participate in histamine H4 receptor-induced itch. Neural Plast 2016:1682972
Villarino AV, Kanno Y, O’Shea JJ (2017) Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol 18:374–384
Wang F, Morris C, Bodet ND, Kim BS (2019) Treatment of refractory chronic pruritus of unknown origin with tofacitinib in patients with rheumatoid arthritis. JAMA Dermatol 155(12):1426–1428
Salmon JA, Higgs GA (1987) Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull 43:285–296
O’Byrne PM, Israel E, Drazen JM (1997) Antileukotrienes in the treatment of asthma. Ann Intern Med 127:472–480
Luster AD, Tager AM (2004) T-cell trafficking in asthma: lipid mediators grease the way. Nat Rev Immunol 4:711–724
Andoh T, Kuraishi Y (1998) Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. Eur J Pharmacol 353:93–96
Fernandes ES, Vong CT, Quek S, Cheong J, Awal S, Gentry C, Aubdool AA, Liang L, Bodkin JV, Bevan S, Heads R, Brain SD (2013) Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B(4)-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J 27:1664–1673
Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, Chesler AT, Hoon MA (2019) Nppb neurons are sensors of mast cell-induced itch. Cell Rep 26:3561–3573.e4
Voisin T, Perner C, Messou MA, Shiers S, Ualiyeva S, Kanaoka Y, Price TJ, Sokol CL, Bankova LG, Austen KF, Chiu IM (2021) The CysLT2R receptor mediates leukotriene C4-driven acute and chronic itch. Proc Natl Acad Sci U S A 118(13):e2022087118
Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ (2002) p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36:57–68
Cheng JK, Ji RR (2008) Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res 33:1970–1978
Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, Liu Q (2019) TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun 10:5678
Yu G, Yang N, Li F, Chen M, Guo CJ, Wang C, Hu D, Yang Y, Zhu C, Wang Z, Shi H, Gegen T, Tang M, He Q, Liu Q, Tang Z (2016) Enhanced itch elicited by capsaicin in a chronic itch model. Mol Pain 12:1744806916645349
Cao E, Liao M, Cheng Y, Julius D (2013) TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504:113–118
Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R (2007) The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54:905–918
Salzer I, Ray S, Schicker K, Boehm S (2019) Nociceptor signalling through ion channel regulation via GPCRs. Int J Mol Sci 20(10):2488
Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ (2005) A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A 102:4536–4541
Chung MK, Guler AD, Caterina MJ (2008) TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci 11:555–564
Touska F, Marsakova L, Teisinger J, Vlachova V (2011) A “cute” desensitization of TRPV1. Curr Pharm Biotechnol 12:122–129
Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D (2013) TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77:667–679
Sun X, Zakharian E (2015) Regulation of the temperature-dependent activation of transient receptor potential vanilloid 1 (TRPV1) by phospholipids in planar lipid bilayers. J Biol Chem 290:4741–4747
Xiao R, Xu XZS (2021) Temperature sensation: from molecular thermosensors to neural circuits and coding principles. Annu Rev Physiol 83:205–230
Mohapatra DP, Nau C (2003) Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem 278:50080–50090
Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ (2006) The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain 2:22
Spahn V, Fischer O, Endres-Becker J, Schafer M, Stein C, Zollner C (2013) Opioid withdrawal increases transient receptor potential vanilloid 1 activity in a protein kinase A-dependent manner. Pain 154:598–608
Baron EP, Lucas P, Eades J, Hogue O (2018) Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain 19:37
Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA (2018) Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 21:695–714
Arnold WR, Carnevale LN, Xie Z, Baylon JL, Tajkhorshid E, Hu H, Das A (2021) Anti-inflammatory dopamine- and serotonin-based endocannabinoid epoxides reciprocally regulate cannabinoid receptors and the TRPV1 channel. Nat Commun 12:926
Por ED, Samelson BK, Belugin S, Akopian AN, Scott JD, Jeske NA (2010) PP2B/calcineurin-mediated desensitization of TRPV1 does not require AKAP150. Biochem J 432:549–556
Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U (2004) Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem 279:7048–7054
Zhang X, Daugherty SL, de Groat WC (2011) Activation of CaMKII and ERK1/2 contributes to the time-dependent potentiation of Ca2+ response elicited by repeated application of capsaicin in rat DRG neurons. Am J Physiol Regul Integr Comp Physiol 300:R644–R654
Ugawa S, Ueda T, Yamamura H, Shimada S (2005) In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Brain Res Mol Brain Res 136:125–133
Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X (2008) Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 133:475–485
Wang C, Gu L, Ruan Y, Gegen T, Yu L, Zhu C, Yang Y, Zhou Y, Yu G, Tang Z (2018) Pirt together with TRPV1 is involved in the regulation of neuropathic pain. Neural Plast 2018:4861491
Wang C, Wang Z, Yang Y, Zhu C, Wu G, Yu G, Jian T, Yang N, Shi H, Tang M, He Q, Lan L, Liu Q, Guan Y, Dong X, Duan J, Tang Z (2015) Pirt contributes to uterine contraction-induced pain in mice. Mol Pain 11:57
Patel KN, Liu Q, Meeker S, Undem BJ, Dong X (2011) Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PLoS One 6:e20559
Hanack C, Moroni M, Lima WC, Wende H, Kirchner M, Adelfinger L, Schrenk-Siemens K, Tappe-Theodor A, Wetzel C, Kuich PH, Gassmann M, Roggenkamp D, Bettler B, Lewin GR, Selbach M, Siemens J (2015) GABA blocks pathological but not acute TRPV1 pain signals. Cell 160:759–770
Shimada SG, LaMotte RH (2008) Behavioral differentiation between itch and pain in mouse. Pain 139:681–687
Akiyama T, Carstens MI, Carstens E (2010) Differential itch- and pain-related behavioral responses and micro-opoid modulation in mice. Acta Derm Venereol 90:575–581
Fu K, Qu L, Shimada SG, Nie H, LaMotte RH (2014) Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neurosci Lett 579:190–194
Sikand P, Shimada SG, Green BG, LaMotte RH (2009) Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 144:66–75
Sikand P, Shimada SG, Green BG, LaMotte RH (2011) Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain 152:2485–2494
Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E (2014) Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 155:80–92
Brumovsky P, Watanabe M, Hokfelt T (2007) Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience 147:469–490
Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF (2009) Cellular basis of itch sensation. Science 325:1531–1534
Carstens EE, Carstens MI, Simons CT, Jinks SL (2010) Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport 21:303–308
Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q (2010) VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68:543–556
Achanta S, Chintagari NR, Brackmann M, Balakrishna S, Jordt SE (2018) TRPA1 and CGRP antagonists counteract vesicant-induced skin injury and inflammation. Toxicol Lett 293:140–148
McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X (2015) Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519:237–241
Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, Oetjen LK, Wang F, Kim BS, Dong X (2019) Activation of mast-cell-expressed Mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 50:1163–1171.e5
Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, Reber LL, Marichal T, Starkl P, Cenac N, Dong X, Tsai M, Galli SJ, Gaudenzio N (2019) House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol 20:1435–1443
Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ (2013) Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52–57
Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, Yipp BG, Liberles SD, Chiu IM (2018) Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat Med 24:417–426
Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, Chiu IM (2018) Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 173:1083–1097.e22
Spits H, Di Santo JP (2011) The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 12:21–27
McKenzie AN (2014) Type-2 innate lymphoid cells in asthma and allergy. Ann Am Thorac Soc 11(Suppl 5):S263–S270
Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, Laedermann C, Foster SL, Tran JV, Lai N, Chiu IM, Ghasemlou N, DiBiase M, Roberson D, Von Hehn C, Agac B, Haworth O, Seki H, Penninger JM, Kuchroo VK, Bean BP, Levy BD, Woolf CJ (2015) Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87:341–354
Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, Veiga-Fernandes H (2017) Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549:277–281
Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, Haas BJ, Tickle TL, Trombetta JJ, Baral P, Klose CSN, Mahlakoiv T, Artis D, Rozenblatt-Rosen O, Chiu IM, Levy BD, Kowalczyk MS, Regev A, Kuchroo VK (2017) The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549:351–356
Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, Shen X, Kostenis E, Konig GM, Senda T, Carpenter D, Farber DL, Artis D (2017) The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549:282–286
Inclan-Rico JM, Ponessa JJ, Valero-Pacheco N, Hernandez CM, Sy CB, Lemenze AD, Beaulieu AM, Siracusa MC (2020) Basophils prime group 2 innate lymphoid cells for neuropeptide-mediated inhibition. Nat Immunol 21:1181–1193
Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM (2013) Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502:245–248
Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH (2015) Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43:515–526
Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, Camire RB, Chow OA, Chiu IM, Sokol CL (2020) Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity 53:1063–1077.e7
Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, Li Y, Zhang S, Ho J, Davis BM, Albers KM, Kaplan DH (2019) Cutaneous TRPV1(+) neurons trigger protective innate type 17 anticipatory immunity. Cell 178:919–932.e14
Anand P, Elsafa E, Privitera R, Naidoo K, Yiangou Y, Donatien P, Gabra H, Wasan H, Kenny L, Rahemtulla A, Misra P (2019) Rational treatment of chemotherapy-induced peripheral neuropathy with capsaicin 8% patch: from pain relief towards disease modification. J Pain Res 12:2039–2052
Cabezon-Gutierrez L, Custodio-Cabello S, Palka-Kotlowska M, Khosravi-Shahi P (2020) High-dose 8% capsaicin patch in treatment of chemotherapy-induced peripheral neuropathy. A systematic review. J Pain Symptom Manage 60:1047–1054.e1
Abrams RMC, Pedowitz EJ, Simpson DM (2021) A critical review of the capsaicin 8% patch for the treatment of neuropathic pain associated with diabetic peripheral neuropathy of the feet in adults. Expert Rev Neurother 21:259–266
Galvez R, Navez ML, Moyle G, Maihofner C, Stoker M, Ernault E, Nurmikko TJ, Attal N (2017) Capsaicin 8% patch repeat treatment in nondiabetic peripheral neuropathic pain: a 52-week, open-label, single-arm, safety study. Clin J Pain 33:921–931
Ruivo EFM, Gestosa SVS, Mulenas NME, Lares AMG (2020) Peripheral neuropathy associated with hypereosinophilic syndrome: a clinical therapeutic success with capsaicin 8% patch. J Pain Palliat Care Pharmacother 34:155–158
Campbell JN, Stevens R, Hanson P, Connolly J, Meske DS, Chung MK, Lascelles BDX (2021) Injectable capsaicin for the management of pain due to osteoarthritis. Molecules 26(4):778
Rami HK, Thompson M, Stemp G, Fell S, Jerman JC, Stevens AJ, Smart D, Sargent B, Sanderson D, Randall AD, Gunthorpe MJ, Davis JB (2006) Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett 16:3287–3291
Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM, Appleby JM (2007) The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 132:132–141
Garami A, Shimansky YP, Rumbus Z, Vizin RCL, Farkas N, Hegyi J, Szakacs Z, Solymar M, Csenkey A, Chiche DA, Kapil R, Kyle DJ, Van Horn WD, Hegyi P, Romanovsky AA (2020) Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: insights from mathematical modeling and meta-analysis. Pharmacol Ther 208:107474
Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210
Manitpisitkul P, Mayorga A, Shalayda K, De Meulder M, Romano G, Jun C, Moyer JA (2015) Safety, tolerability and pharmacokinetic and pharmacodynamic learnings from a double-blind, randomized, placebo-controlled, sequential group first-in-human study of the TRPV1 antagonist, JNJ-38893777, in healthy men. Clin Drug Investig 35:353–363
Srour J, Bengel J, Linden T, Jovanovic Z, Roggenkamp D, Reinholz M, Rothenberger C, Neufang G, Wollenberg A (2020) Efficacy of a skin care cream with TRPV1 inhibitor 4-t-butylcyclohexanol in the topical therapy of perioral dermatitis. J Cosmet Dermatol 19:1409–1414
Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ (2005) AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313:474–484
Norman MH, Zhu J, Fotsch C, Bo Y, Chen N, Chakrabarti P, Doherty EM, Gavva NR, Nishimura N, Nixey T, Ognyanov VI, Rzasa RM, Stec M, Surapaneni S, Tamir R, Viswanadhan VN, Treanor JJ (2007) Novel vanilloid receptor-1 antagonists: 1. Conformationally restricted analogues of trans-cinnamides. J Med Chem 50:3497–3514
Huang CC, Kim YS, Olson WP, Li F, Guo C, Luo W, Huang AJW, Liu Q (2016) A histamine-independent itch pathway is required for allergic ocular itch. J Allergy Clin Immunol 137:1267–1270.e6
Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB (2018) Regulation of pain and itch by TRP channels. Neurosci Bull 34:120–142
Binshtok AM, Bean BP, Woolf CJ (2007) Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449:607–610
Blake KJ, Baral P, Voisin T, Lubkin A, Pinho-Ribeiro FA, Adams KL, Roberson DP, Ma YC, Otto M, Woolf CJ, Torres VJ, Chiu IM (2018) Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat Commun 9:37
Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ (2013) Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci 16:910–918
Huang CC, Yang W, Guo C, Jiang H, Li F, Xiao M, Davidson S, Yu G, Duan B, Huang T, Huang AJW, Liu Q (2018) Anatomical and functional dichotomy of ocular itch and pain. Nat Med 24:1268–1276
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Li, F., Wang, F. (2021). TRPV1 in Pain and Itch. In: Zhou, L. (eds) Ion Channels in Biophysics and Physiology. Advances in Experimental Medicine and Biology, vol 1349. Springer, Singapore. https://doi.org/10.1007/978-981-16-4254-8_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-4254-8_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4253-1
Online ISBN: 978-981-16-4254-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)