Abstract
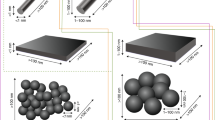
Since the year 2000, nanotechnology has been an emerging factor in science, economy, and also daily life. Although nano size was well known in the past in chemistry (e.g., kolloids, catalysts), due to the huge number of engineered synthetic nanomaterials and their wide range of technical applications, a new broad economic field has come up. Regulatory bodies developed strategies to include nanomaterials into the existing legislation of chemicals, biocides, and food additives. This process was facilitated by the fact that principally not the chemistry changed from micro- to nanoscale but mainly physico-chemical properties (specific surface, solubility, agglomeration status, etc.). Thus, toxicological effects were not expected to be principally different; however, the evaluation had to be expanded from target organ effects (e.g., lungs, skin) to potential systemic effects. Due to the tiny size and an increased dissolution of nanoparticles, the toxicokinetics became a predominant additional endpoint. This summary is based on the technical guidelines, decisions, and laws issued by the main regulatory bodies such as the Organisation for Economic Co-operation and Development (OECD), European Chemicals Agency (ECHA), European Food Safety Authority (EFSA), European Medicines Agency (EMA), U.S. Food and Drug Administration (FDA), and U.S. Environmental Protection Agency (EPA). This chapter is presenting the regulatory status of nanomaterials being aware of the still ongoing process.
Similar content being viewed by others
References
Arts JHE, Hadib M, Irfanc M-A et al (2015) A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul Toxicol Pharmacol 71:S1–S27. https://doi.org/10.1016/j.yrtph.2015.03.007
BAuA (2018) Justification document for the selection of a CoRAP Substance, 2nd Update 20-Mar-2018
ECHA (2017) Titanium dioxide proposed to be classified as suspected of causing cancer when inhaled. https://echa.europa.eu/de/-/titanium-dioxide-proposed-to-be-classified-as-suspected-of-causing-cancer-when-inhaled. Accessed 10 Dec 2020
ECHA (2018). Nanomaterials in REACH and CLP – Revision of REACH technical annexes. https://ec.europa.eu/environment/chemicals/nanotech/reach-clp/index_en.htm. Accessed 10 Dec 2020
EFSA (2019) EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J 17(6):5714
European Commission (2009) Regulation (ec) no 1223/2009 of the European parliament and of the council of 30 November 2009 on cosmetic products
European Commission (2011) Recommendation on the definition of nanomaterial (2011/696/EU) – 18 October 2011
FDA (2018) Nanotechnology Fact Sheet. 23-Mar-2018
IARC (2017) International agency for research on cancer. In: Some Nanomaterials and some fibres, IARC monographs on the evaluation of carcinogenic risks to humans, vol 111. ISBN 978-92-832-0177-9, May 19th 2017
Jeevanandam J, Barhoum A, Chan YS et al (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Landsiedel R, Ma-Hock L, Kroll A et al (2010) Testing metal-oxide nanomaterials for human safety. Adv Mater 22:2601–2627
Oberdörster G (2010) Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med 267:89–105. https://doi.org/10.1111/j.1365-2796.2009.02187.x
Rasmussen K, Rauscher H, Kearns P et al (2019) Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data. Regul Toxicol Pharmacol 104:74–83 https://doi.org/10.1016/j.yrtph.2019.02.008
Rauscher H et al (2017) Regulatory Aspects of Nanomaterials in the EU. Chem Ing Tech 89:225–231
Resnik DB (2019) How should engineered nanomaterials be regulated for public and environmental health? AMA J Ethics 21(4):E363–E369
Rittinghausen S, Hackbarth A, Creutzenberg O et al (2014) The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fibre Toxicol 11:59
SafeNano (2020) Current activity relating to the regulation of nanotechnologies. https://www.safenano.org/knowledgebase/regulation/. Accessed 10 Dec 2020
Schellekens H, Stegemann S, Weinstein V et al (2014) How to regulate nonbiological complex drugs (NBCD) and their follow-on versions: points to consider. AAPS J 16:15–21. https://doi.org/10.1208/s12248-013-9533-z
Schneider G (2017) Antimicrobial silver nanoparticles – regulatory situation in the European Union. Mater Today Proc 4:S200–S207
Smith B (2018) How is nanotechnology regulated? AZONANO Sept 18, 2018
TDMA (2020) What you should know about EU titanium dioxide regulations. https://tdma.info/what-you-should-know-about-eu-titanium-dioxide-regulations/#:~:text=Es-,What%20you%20should%20know%20about%20EU%20titanium%20dioxide%20(TiO2,or%20used%20within%20the%20EU. Accessed 10 Dec 2020
Yah S, Iyuke SE, Simate GS (2011) A review of nanoparticles toxicity and their routes of exposures. Iran J Pharm Sci 8:299–314
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer-Verlag GmbH Germany, part of Springer Nature
About this entry
Cite this entry
Creutzenberg, O. (2021). Nanoparticles and Their Regulation. In: Reichl, FX., Schwenk, M. (eds) Regulatory Toxicology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36206-4_122-1
Download citation
DOI: https://doi.org/10.1007/978-3-642-36206-4_122-1
Received:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36206-4
Online ISBN: 978-3-642-36206-4
eBook Packages: Springer Reference Biomedicine and Life SciencesReference Module Biomedical and Life Sciences