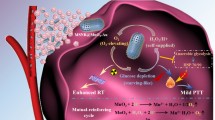
Abstract
Cancer has been globally acknowledged as one of the most dangerous health problems. Although current therapies have shown promising results, they cannot eliminate all tumor cells and prevent the recurrence of these cells in the long term. The aggressive progress of a tumor can be triggered when the cancer is suffered from oxygen depletion or so-called hypoxia. Previously, researchers primarily utilized catalase's enzymatic activity to convert the endogenous hydrogen peroxide in cancer cells into oxygen, alleviating intracellular hypoxia. However, using natural enzymes has faced limitations, such as low stability and uneasy and costly production, making it hard to commercialize this method. To tackle the problem, synthetic nanomaterials, which could mimic enzyme-like activity, are utilized and named nanozyme. The ability of nanozymes mostly comes from the redox reaction of cations of high-Z metals, such as manganese, iron, cerium, and iridium. This review will offer readers an overview of the synergistic effect of nanozyme-based delivery models with the ability to generate oxygen to attenuate tumor hypoxia and enhance the therapeutic outcome. Based on the literature search, each type of nanozymes possesses both pros and cons; however, they all perform well in suppressing tumor hypoxia and improving therapeutic efficacy. Though, more studies must be conducted to guarantee these catalase-mimicking nanozymes’ long-term safety before the clinical application. Besides, solving the problem of hypoxia also opens up new paths for potential applications in 3D in-vitro cancer cell culture.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sung, H., et al.: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021). https://doi.org/10.3322/CAAC.21660
Jing, X., et al.: Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 18(1), 1–15 (2019). https://doi.org/10.1186/s12943-019-1089-9
Sun, Y.: Tumor microenvironment and cancer therapy resistance. Cancer Lett. 380(1), 205–215 (2016). https://doi.org/10.1016/j.canlet.2015.07.044
Quail, D.F., Joyce, J.A.: Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19(11), 1423–1437 (2013). https://doi.org/10.1038/nm.3394
Tanaka, K., et al.: MiR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis 36(8), 894–903 (2015). https://doi.org/10.1093/CARCIN/BGV067
Özdemir, B.C., et al.: Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25(6), 719–734 (2014). https://doi.org/10.1016/j.ccr.2014.04.005
Rhim, A.D., et al.: Stromal elements act to restrain, rather than support. Pancreatic Ductal Adenocarcinoma. Cancer Cell 25(6), 735–747 (2014). https://doi.org/10.1016/j.ccr.2014.04.021
Duluc, C., et al.: Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol. Med. 7(6), 735–753 (2015). https://doi.org/10.15252/emmm.201404346
Erez, N., Truitt, M., Olson, P., Hanahan, D.: Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17(2), 135–147 (2010). https://doi.org/10.1016/j.ccr.2009.12.041
Erez, N., Glanz, S., Raz, Y., Avivi, C., Barshack, I.: Cancer Associated Fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 437(3), 397–402 (2013). https://doi.org/10.1016/j.bbrc.2013.06.089
Nagasaki, T., Hara, M., Nakanishi, H., Takahashi, H., Sato, M., Takeyama, H.: Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour–stroma interaction. Br. J. Cancer 110(2), 469–478 (2014). https://doi.org/10.1038/bjc.2013.748
Zhang, X.H.-F., et al.: Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154(5), 1060–1073 (2013). https://doi.org/10.1016/j.canlet.2015.07.044
Song, T., et al.: TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget 6(14), 12061–12079 (2015). https://doi.org/10.18632/oncotarget.3616
Qiu, G.Z., Jin, M.Z., Dai, J.X., Sun, W., Feng, J.H., Jin, W.L.: Reprogramming of the tumor in the hypoxic niche: the emerging concept and associated therapeutic strategies. Trends Pharmacol. Sci. 38(8), 669–686 (2017). https://doi.org/10.1016/j.tips.2017.05.002
Schito, L., Semenza, G.L.: Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2(12), 758–770 (2016). https://doi.org/10.1016/j.trecan.2016.10.016
Majmundar, A.J., Wong, W.J., Simon, M.C.: Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40(2), 294–309 (2010). https://doi.org/10.1016/j.molcel.2010.09.022
Roma-Rodrigues, C., Mendes, R., Baptista, P.V., Fernandes, A.R.: Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 20(4), 840 (2019). https://doi.org/10.3390/ijms20040840
Wang, B., et al.: Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy. J. Exp. Clin. Cancer Res. 40(1), 1–16 (2021). https://doi.org/10.1186/s13046-020-01820-7
Noman, M.Z., et al.: Improving cancer immunotherapy by targeting the hypoxic tumor microenvironment: new opportunities and challenges. Cells 8(9), 1083 (2019). https://doi.org/10.3390/cells8091083
Zhang, R., et al.: Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials 138, 13–21 (2017). https://doi.org/10.1016/j.biomaterials.2017.05.025
Phua, S.Z.F., et al.: Catalase-integrated hyaluronic acid as nano carriers for enhanced photodynamic therapy in solid tumor. ACS Nano 13(4), 4742–4751 (2019). https://doi.org/10.1021/acsnano.9b01087
Alfonso-Prieto, M., Biarnés, X., Vidossich, P., Rovira, C.: The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 131(33), 11751–11761 (2009). https://doi.org/10.1021/ja9018572
Chelikani, P., Fita, I., Loewen, P.C.: Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61(2), 192–208 (2004). https://doi.org/10.1007/s00018-003-3206-5
Liang, M., Yan, X.: Nanozymes: from new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 52(8), 2190–2200 (2019). https://doi.org/10.1021/acs.accounts.9b00140
Jiang, D., Ni, D., Rosenkrans, Z.T., Huang, P., Yan, X., Cai, W.: Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 48(14), 3683–3704 (2019). https://doi.org/10.1039/c8cs00718g
Zhang, Y., Jin, Y., Cui, H., Yan, X., Fan, K.: Nanozyme-based catalytic theranostics. RSC Adv. 10(1), 10–20 (2020). https://doi.org/10.1039/c9ra09021e
Gao, L., Yan, X.: Nanozymes: an emerging field bridging nanotechnology and biology. Sci. China Life Sci. 59(4), 400–402 (2016). https://doi.org/10.1007/s11427-016-5044-3
Maji, S.K., Mandal, A.K., Nguyen, K.T., Borah, P., Zhao, Y.: Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanoparticle-loaded mesoporous silica-coated graphene. ACS Appl. Mater. Interfaces 7(18), 9807–9816 (2015). https://doi.org/10.1021/acsami.5b01758
Meng, L., et al.: Tumor oxygenation and hypoxia inducible factor-1 functional inhibition via a reactive oxygen species responsive nanoplatform for enhancing radiation therapy and abscopal effects. ACS Nano 12(8), 8308–8322 (2018). https://doi.org/10.1021/acsnano.8b03590
Salim, S. A., Salaheldin, T. A., Elmazar, M. M., Abdel-Aziz, A. F., Kamoun, E. A.: Smart biomaterials for enhancing cancer therapy by overcoming tumor hypoxia: a review. RSC Adv. 12(52), 33835–33851 (2022). https://doi.org/10.1039/d2ra06036a
Sahu, A., Kwon, I., Tae, G.: Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials 228, 119578 (2020). https://doi.org/10.1016/j.biomaterials.2019.119578
Meng, L., et al.: Facile deposition of manganese dioxide to albumin-bound paclitaxel nanoparticles for modulation of hypoxic tumor microenvironment to improve chemoradiation therapy. Mol. Pharm. 15(2), 447–457 (2018). https://doi.org/10.1021/acs.molpharmaceut.7b00808
Elzoghby, A.O., Samy, W.M., Elgindy, N.A.: Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 157(2), 168–182 (2012). https://doi.org/10.1016/j.jconrel.2011.07.031
Milas, L., Hunter, N.R., Mason, K.A., Kurdoglu, B., Peters, L.J.: Enhancement of tumor radioresponse of a murine mammary carcinoma by paclitaxel. Cancer Res. 54(13), 3506–3510 (1994). PMID: 7912167
Safran, H., et al.: Paclitaxel and concurrent radiation for locally advanced pancreatic and gastric cancer: a phase I study. J. Clin. Oncol. 15(3), 901–907 (1997). https://doi.org/10.1200/JCO.1997.15.3.901
Rich, T., et al.: Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-98–12. Am. J. Clin. Oncol. Cancer Clin. Trials 27(1), 51–56 (2004). https://doi.org/10.1097/01.coc.0000046300.88847.BF
Mikhail, A.S., Allen, C.: Block copolymer micelles for delivery of cancer therapy: transport at the whole body, tissue and cellular levels. J. Control. Release 138(3), 214–223 (2009). https://doi.org/10.1016/j.jconrel.2009.04.010
Minchinton, A.I., Tannock, I.F.: Drug penetration in solid tumours. Nat. Rev. Cancer 6(8), 583–592 (2006). https://doi.org/10.1038/nrc1893
Chen, Q., et al.: Intelligent Albumin–MnO2 Nanoparticles as pH-/H2O2-Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy. Adv. Mater. 28(33), 7129–7136 (2016). https://doi.org/10.1002/adma.201601902
Tang, L., Gabrielson, N.P., Uckun, F.M., Fan, T.M., Cheng, J.: Size-dependent tumor penetration and in vivo efficacy of monodisperse drug-silica nanoconjugates. Mol. Pharm. 10(3), 883–892 (2013). https://doi.org/10.1021/mp300684a
Popović, Z., et al.: A Nanoparticle size series for in vivo fluorescence imaging. Angew. Chemie 122(46), 8831–8834 (2010). https://doi.org/10.1002/ange.201003142
Yuan, F., et al.: Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 55(17), 3752–3756 (1995). ISSN:0008-5472
Jain, R.K.: Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706), 58–62 (2005). https://doi.org/10.1126/science.1104819
Perrault, S.D., Walkey, C., Jennings, T., Fischer, H.C., Chan, W.C.W.: Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 9(5), 1909–1915 (2009). https://doi.org/10.1021/nl900031y
Stowe, D.F., Camara, A.K.S.: Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid. Redox Signal. 11(6), 1373–1414 (2009). https://doi.org/10.1089/ars.2008.2331
Kavčič, N., Pegan, K., Vandenabeele, P., Turk, B.: Comparative study of the differential cell death protecting effect of various ROS scavengers. Biol. Chem. 400(2), 149–160 (2019). https://doi.org/10.1515/hsz-2017-0317
Moloney, J.N., Cotter, T.G.: ROS signaling in the biology of cancer. Semin. Cell Dev. Biol. 80, 50–64 (2018). https://doi.org/10.1016/j.semcdb.2017.05.023
Kiesslich, T., Plaetzer, K., Oberdanner, C.B., Berlanda, J., Obermair, F.J., Krammer, B.: Differential effects of glucose deprivation on the cellular sensitivity towards photodynamic treatment-based production of reactive oxygen species and apoptosis-induction. FEBS Lett. 579(1), 185–190 (2005). https://doi.org/10.1016/j.febslet.2004.11.073
Hall, M.D., Hambley, T.W.: Platinum(IV) antitumour compounds: their bioinorganic chemistry. Coord. Chem. Rev. 232(1–2), 49–67 (2002). https://doi.org/10.1016/S0010-8545(02)00026-7
Homolya, Váradi, A., Sarkadi, B.: Multidrug resistance-associated proteins: Export pumps for conjugates with glutathione, glucuronate or sulfate. Biofactors 17(1–4), 103–114 (2003). https://doi.org/10.1002/biof.5520170111
Mellish, K., Kelland, L., Harrap, K.: In vitro platinum drug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br. J. Cancer 68(2), 240–250 (1993). https://doi.org/10.1038/bjc.1993.322
Liu, P., Xie, X., Liu, M., Hu, S., Ding, J., Zhou, W.: A smart MnO2-doped graphene oxide nanosheet for enhanced chemo-photodynamic combinatorial therapy via simultaneous oxygenation and glutathione depletion. Acta Pharm. Sin. B 11(3), 823–834 (2021). https://doi.org/10.1016/j.apsb.2020.07.021
Zhang, H., Grüner, G., Zhao, Y.: Recent advancements of graphene in biomedicine. J. Mater. Chem. B 1(20), 2542–2567 (2013). https://doi.org/10.1039/c3tb20405g
Liu, Y., Yang, G., Jin, S., Xu, L., Zhao, C.-X.: Development of high-drug-loading nanoparticles. ChemPlusChem 85(9), 2143–2157 (2020). https://doi.org/10.1002/cplu.202000496
Zhou, Z., et al.: Ping: overcoming chemotherapy resistance using pH-sensitive hollow MnO2 nanoshells that target the hypoxic tumor microenvironment of metastasized oral squamous cell carcinoma. J. Nanobiotechnology 19(1), 157 (2021). https://doi.org/10.1186/s12951-021-00901-9
Li, Y., Shi, J.: Hollow-structured mesoporous materials: chemical synthesis, functionalization and applications. Adv. Mater. 26(20), 3176–3205 (2014). https://doi.org/10.1002/adma.201305319
Cheng, M., et al.: Monodisperse hollow MnO2 with biodegradability for efficient targeted drug delivery. ACS Biomater. Sci. Eng. 6(9), 4985–4992 (2020). https://doi.org/10.1021/acsbiomaterials.0c00507
Wu, M., et al.: Manganese dioxide nanosheets: from preparation to biomedical applications. Int. J. Nanomedicine 14, 4781–4800 (2019). https://doi.org/10.2147/IJN.S207666
Zhao, M., Huang, Y., Peng, Y., Huang, Z., Ma, Q., Zhang, H.: Two-dimensional metal–organic framework nanosheets: synthesis and applications. Chem. Soc. Rev. 47(16), 6267–6295 (2018). https://doi.org/10.1039/c8cs00268a
Liu, Z., et al.: Theranostic 2D ultrathin MnO2 nanosheets with fast responsibility to endogenous tumor microenvironment and exogenous NIR irradiation. Biomaterials 155, 54–63 (2018). https://doi.org/10.1016/j.biomaterials.2017.11.015
Gao, S., Lin, H., Zhang, H., Yao, H., Chen, Y., Shi, J.: Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv. Sci. 6(3), 1801733 (2019). https://doi.org/10.1002/advs.201801733
Finkel, T., Serrano, M., Blasco, M.A.: The common biology of cancer and ageing. Nature 448(7155), 767–774 (2007). https://doi.org/10.1038/nature05985
Wang, J., et al.: Inorganic nanozyme with combined self-oxygenation/degradable capabilities for sensitized cancer immunochemotherapy. Nano-Micro Lett. 11(1), 1–18 (2019). https://doi.org/10.1007/s40820-019-0305-x
Liu, X., et al.: Gold nanoparticles doped metal-organic frameworks as near-infrared light-enhanced cascade nanozyme against hypoxic tumors. Nano Res. 13(3), 653–660 (2020). https://doi.org/10.1007/s12274-020-2668-1
He, C., et al.: A solid lipid coated calcium peroxide nanocarrier enables combined cancer chemo/chemodynamic therapy with O2/H2O2 self-sufficiency. Acta Biomater. 122, 354–364 (2021). https://doi.org/10.1016/j.actbio.2020.12.036
Pham, A.L.T., Lee, C., Doyle, F.M., Sedlak, D.L.: A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environ. Sci. Technol. 43(23), 8930–8935 (2009). https://doi.org/10.1021/es902296k
Mamat, M., et al.: CaO2/Fe3O4 nanocomposites for oxygen-independent generation of radicals and cancer therapy. Colloids Surf. B 204, 111803 (2021). https://doi.org/10.1016/j.colsurfb.2021.111803
Korsvik, C., Patil, S., Seal, S., Self, W.T.: Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 10, 1056–1058 (2007). https://doi.org/10.1039/b615134e
Pirmohamed, T., et al.: Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 46(16), 2736–2738 (2010). https://doi.org/10.1039/b922024k
Zeng, L., et al.: In vivo regenerable cerium oxide nanozyme-loaded pH/H2O2-responsive nanovesicle for tumor-targeted photothermal and photodynamic therapies. ACS Appl. Mater. Interfaces 13(1), 233–244 (2021). https://doi.org/10.1021/acsami.0c19074
Fan, Y., et al.: A smart photosensitizer-cerium oxide nanoprobe for highly selective and efficient photodynamic therapy. Inorg. Chem. 58(11), 7295–7302 (2019). https://doi.org/10.1021/acs.inorgchem.9b00363
Wei, C., et al.: Iridium/ruthenium nanozyme reactors with cascade catalytic ability for synergistic oxidation therapy and starvation therapy in the treatment of breast cancer. Biomaterials 238, 119848 (2020). https://doi.org/10.1016/j.biomaterials.2020.119848
Feng, L., et al.: Iridium nanocrystals encapsulated liposomes as near-infrared light controllable nanozymes for enhanced cancer radiotherapy. Biomaterials 181, 81–91 (2018). https://doi.org/10.1016/j.biomaterials.2018.07.049
Yuan, X., et al.: Iridium oxide nanoparticles mediated enhanced photodynamic therapy combined with photothermal therapy in the treatment of breast cancer. J. Colloid Interface Sci. 605, 851–862 (2022). https://doi.org/10.1016/j.jcis.2021.07.136
Armstrong, F.A.: Why did Nature choose manganese to make oxygen? Philos. Trans. R. Soc. B Biol. Sci. 363(1494), 1263–1270 (2008). https://doi.org/10.1098/rstb.2007.2223
De Witt Hamer, P.C., et al.: The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene 27(14), 2091–2096 (2008). https://doi.org/10.1038/sj.onc.1210850
Sharifi, S., Behzadi, S., Laurent, S., Forrest, M.L., Stroeve, P., Mahmoudi, M.: Toxicity of nanomaterials. Chem. Soc. Rev. 41(6), 2323–2343 (2012). https://doi.org/10.1039/c1cs15188f
Acknowledgments
This work was supported by International University (Grant no: T2020-01-BT).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Nguyen, D.N., Nguyen, K.T. (2024). Catalase-Like Nanozymes and Their Applications in Alleviating Tumor Hypoxia for the Therapeutic Enhancement. In: Vo, V.T., Nguyen, TH., Vong, B.L., Le, N.B., Nguyen, T.Q. (eds) 9th International Conference on the Development of Biomedical Engineering in Vietnam. BME 2022. IFMBE Proceedings, vol 95. Springer, Cham. https://doi.org/10.1007/978-3-031-44630-6_25
Download citation
DOI: https://doi.org/10.1007/978-3-031-44630-6_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44629-0
Online ISBN: 978-3-031-44630-6
eBook Packages: EngineeringEngineering (R0)