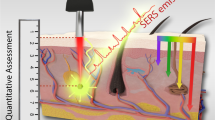
Abstract
In the field of clinical biology, imaging biological tissues play a crucial role in understanding the nature of structural and physiological changes in their content. Traditionally, for physiological and morphological studies of infected tissues, two-dimensional surface imaging of tissue sections was the standard. However, in many situations, 3D imaging of an entire tissue region becomes mandatory for a better understanding of the condition. This requires an imaging modality that can image deep inside the tissues with better resolution. But animal tissues are notorious for scattering visible light thus making it difficult to see any anomalies beyond 100 μm depth. To decrease the scattering by the tissue, IR light can be used. But according to Abbe’s law, the resolution of a microscope system is inversely proportional to the wavelength of light used. So, as two-photon excitation uses approximately twice the one-photon wavelength, the resolution becomes approximately half of the single photon. Moreover, as we try to observe deeper, scattering makes it difficult to cope with the decrease in the contrast between the object of interest and the unwanted part of the tissue. In this chapter, we will discuss one recently developed technique called Saturation Excitation (SAX) microscopy to achieve improved resolution and background-free images from depths of animal tissues using nonlinear plasmonic nanoparticle scattering as markers. Herein, we will discuss the working principle of the SAX technique and review its potential in imaging as deep as 400 μm with improved resolution and background-free contrast.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
R.M. Kwee, T.C. Kwee, Imaging in local staging of gastric cancer: A systematic review. J. Clin. Oncol. 25(15), 2107–2116 (2007). https://doi.org/10.1200/JCO.2006.09.5224
L. Fass, Imaging and cancer: A review. Mol. Oncol. 2(2), 115–152 (2008). https://doi.org/10.1016/j.molonc.2008.04.001
C. Cremer et al., Superresolution imaging of biological nanostructures by spectral precision distance microscopy. Biotechnol. J. 6(9), 1037–1051 (2011). https://doi.org/10.1002/biot.201100031
F. Helmchen, W. Denk, Deep tissue two-photon microscopy. Nat. Methods 2(12), 932–940 (2005). https://doi.org/10.1038/nmeth818
D.R. Miller, J.W. Jarrett, A.M. Hassan, A.K. Dunn, Deep tissue imaging with multiphoton fluorescence microscopy. Curr. Opin. Biomed. Eng. 4, 32–39 (2017). https://doi.org/10.1016/j.cobme.2017.09.004
A. Feuchtinger, A. Walch, M. Dobosz, Deep tissue imaging: a review from a preclinical cancer research perspective. Histochem. Cell Biol. 146(6), 781–806 (2016). https://doi.org/10.1007/s00418-016-1495-7
S.-G. Kim, W. Richter, K. Uğurbil, Limitations of temporal resolution in functional MRI. Magn. Reson. Med. 37(4), 631–636 (1997). https://doi.org/10.1002/mrm.1910370427
A. Pizurica, A. Wink, E. Vansteenkiste, W. Philips, B.J. Roerdink, A review of wavelet denoising in MRI and ultrasound brain imaging. Curr. Med. Imaging Rev. 2(2), 247–260 (2006). https://doi.org/10.2174/157340506776930665
W. Denk, J.H. Strickler, W.W. Webb, Two-photon laser scanning fluorescence microscopy. Science 248(4951), 73–76 (1990). https://doi.org/10.1126/science.2321027
D.G. Ouzounov et al., In vivo three-photon imaging of activity of GcamP6-labeled neurons deep in intact mouse brain. Nat. Methods 14(4), 388–390 (2017). https://doi.org/10.1038/nmeth.4183
R.K.P. Benninger, D.W. Piston, Two-photon excitation microscopy for unit 4.11 the study of living cells and tissues. Curr. Protoc. Cell Biol. 59(1), 4–11 (2013). https://doi.org/10.1002/0471143030.cb0411s59
G. Moneron, S.W. Hell, Two-photon excitation STED microscopy. Opt. Express 17(17), 14567 (2009). https://doi.org/10.1364/oe.17.014567
M. Ingaramo et al., Two-photon excitation improves multifocal structured illumination microscopy in thick scattering tissue. Proc. Natl. Acad. Sci. U. S. A. 111(14), 5254–5259 (2014). https://doi.org/10.1073/pnas.1314447111
K. Temprine, A.G. York, H. Shroff, Three-dimensional photoactivated localization microscopy with genetically expressed probes. Methods Mol. Biol. 1251, 231–261 (2014). https://doi.org/10.1007/978-1-4939-2080-8_13
G. Vicidomini, P. Bianchini, A. Diaspro, STED super-resolved microscopy. Nat. Methods 15(3), 173–182 (2018). https://doi.org/10.1038/nmeth.4593
R. Sharma, M. Singh, R. Sharma, Recent advances in STED and RESOLFT super-resolution imaging techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc. 231, 117715 (2020). https://doi.org/10.1016/j.saa.2019.117715
K. Fujita, M. Kobayashi, S. Kawano, M. Yamanaka, S. Kawata, High-resolution confocal microscopy by saturated excitation of fluorescence. Phys. Rev. Lett. 99(22), 228105 (2007). https://doi.org/10.1103/PhysRevLett.99.228105
S.W. Chu et al., Measurement of a saturated emission of optical radiation from gold nanoparticles: Application to an ultrahigh resolution microscope. Phys. Rev. Lett. 112(1), 017402 (2014). https://doi.org/10.1103/PhysRevLett.112.017402
S.W. Chu et al., Saturation and reverse saturation of scattering in a single plasmonic nanoparticle. ACS Photonics 1(1), 32–37 (2014). https://doi.org/10.1021/ph4000218
Y.T. Chen et al., Study of nonlinear plasmonic scattering in metallic nanoparticles. ACS Photonics 3(8), 1432–1439 (2016). https://doi.org/10.1021/acsphotonics.6b00025
A.V. Zayats, I.I. Smolyaninov, A.A. Maradudin, Nano-optics of surface plasmon polaritons. Phys. Rep. 408(3–4), 131–314 (2005). https://doi.org/10.1016/j.physrep.2004.11.001
K.A. Willets, A.J. Wilson, V. Sundaresan, P.B. Joshi, Super-resolution imaging and plasmonics. Chem. Rev. 117(11), 7538–7582 (2017). https://doi.org/10.1021/acs.chemrev.6b00547
Y.C. Ding, P. Xi, Q.S. Ren, Hacking the optical diffraction limit: Review on recent developments of fluorescence nanoscopy. Chinese Sci. Bull. 56(18), 1857–1876 (2011). https://doi.org/10.1007/s11434-011-4502-3
G.R. Bullock, The current status of fixation for electron microscopy: A review. J. Microsc. 133(1), 1–15 (1984). https://doi.org/10.1111/j.1365-2818.1984.tb00458.x
A. Mohammed, A. Abdullah, Scanning electron microscopy (SEM): A review, (2019)
A. Stemmer, M. Beck, R. Fiolka, Widefield fluorescence microscopy with extended resolution. Histochem. Cell Biol. 130(5), 807–817 (2008). https://doi.org/10.1007/s00418-008-0506-8
M. Minsky, Memoir on inventing the confocal scanning microscope. Scanning 10(4), 128–138 (1988). https://doi.org/10.1002/sca.4950100403
M. Göppert-Mayer, Über Elementarakte mit zwei Quantensprüngen. Ann. Phys. 401(3), 273–294 (1931). https://doi.org/10.1002/andp.19314010303
P.T.C. So, C.Y. Dong, B.R. Masters, K.M. Berland, Two-photon excitation fluorescence microscopy. Annu. Rev. Biomed. Eng., 399–429 (2000)
C.J. Engelbrecht, E.H. Stelzer, Resolution enhancement in a light-sheet-based microscope (SPIM). Opt. Lett. 31(10), 1477 (2006). https://doi.org/10.1364/ol.31.001477
C.G. Galbraith, J.A. Galbraith, Super-resolution microscopy at a glance. J. Cell Sci. 124(10), 1607–1611 (2011). https://doi.org/10.1242/jcs.080085
T.C. Jagadale, S.-W. Chu, Super-Resolution Imaging Based on Nonlinear Plasmonic Scattering, vol 1 (Springer International Publishing, 2019)
Y. De Wilde, P.A. Lemoine, Review of NSOM microscopy for materials. AIP Conf. Proc. 931(1), 43–52 (2007). https://doi.org/10.1063/1.2799414
D. Axelrod, Total internal reflection fluorescence microscopy in cell biology. Traffic 2(11), 764–774 (2001). https://doi.org/10.1034/j.1600-0854.2001.21104.x
R.M. Dickson, A.B. Cubitt, R.Y. Tsien, W.E. Moerner, On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature 388(6640), 355–358 (1997). https://doi.org/10.1038/41048
E. Betzig et al., Imaging intracellular fluorescent proteins at nanometer resolution. Science 313(5793), 1642–1645 (2006). https://doi.org/10.1126/science.1127344
M.J. Rust, M. Bates, X. Zhuang, Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3(10), 793–795 (2006). https://doi.org/10.1038/nmeth929
S.W. Hell, J. Wichmann, Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19(11), 780 (1994). https://doi.org/10.1364/ol.19.000780
M. Yamanaka, N.I. Smith, K. Fujita, Introduction to super-resolution microscopy. Microscopy 63(3), 177–192 (2014). https://doi.org/10.1093/jmicro/dfu007
M. Yamanaka et al., SAX microscopy with fluorescent nanodiamond probes for high-resolution fluorescence imaging. Biomed. Opt. Express 2(7), 1946 (2011). https://doi.org/10.1364/boe.2.001946
M. Yamanaka et al., Saturated excitation microscopy for sub-diffraction-limited imaging of cell clusters. J. Biomed. Opt. 18(12), 1 (2013). https://doi.org/10.1117/1.JBO.18.12.126002
G. Deka, K. Nishida, K. Mochizuki, H.X. Ding, K. Fujita, S.W. Chu, Resolution enhancement in deep-tissue nanoparticle imaging based on plasmonic saturated excitation microscopy. APL Photonics 3(3) (2018). https://doi.org/10.1063/1.5021455
K. Nishida, G. Deka, N.I. Smith, S.W. Chu, K. Fujita, Nonlinear scattering of near-infrared light for imaging plasmonic nanoparticles in deep tissue. ACS Photonics 7(8), 2139–2146 (2020). https://doi.org/10.1021/acsphotonics.0c00607
Handbook of Surface Plasmon Resonance - Google Books. https://books.google.co.in/books?hl=en&lr=&id=bHEoDwAAQBAJ&oi=fnd&pg=PA15&dq=surface+plasmon+external+electric+field&ots=uiTQznh2up&sig=ss4sUp8Y3rhkBPH9DUDVZtfB4SE&redir_esc=y#v=onepage&q=surface.plasmon.external.electric%20field&f=false. Accessed 30 Apr 2021
A. Kawashima et al., Enhanced magneto-optical properties of semiconductor EuS nanocrystals assisted by surface plasmon resonance of gold nanoparticles. Chem. A Eur. J. 19(43), 14438–14445 (2013). https://doi.org/10.1002/chem.201302259
S. Hong, X. Li, Optimal size of gold nanoparticles for surface-enhanced Raman spectroscopy under different conditions. J. Nanomater. 2013 (2013). https://doi.org/10.1155/2013/790323
X. Fan, W. Zheng, D.J. Singh, Light scattering and surface plasmons on small spherical particles. Light Sci. Appl. 3(6), e179–e179 (2014). https://doi.org/10.1038/lsa.2014.60
H.-Y. Wu et al., Ultrasmall all-optical plasmonic switch and its application to superresolution imaging OPEN. Mol. Imaging Cent. 2(1) (2016). https://doi.org/10.1038/srep24293
S. Alizadeh, Z. Nazari, A review on gold nanoparticles aggregation and its applications. J. Chem. Rev. 2(4), 228–242 (2020). https://doi.org/10.22034/JCR.2020.108561
V.V. Tuchin, Light scattering study of tissues. Physics-Uspekhi 40(5), 495–515 (1997). https://doi.org/10.1070/pu1997v040n05abeh000236
J. Sanderson, Theory of contrast control in the microscope, by Jeremy Sanderson. Quekett J. Microsc. (2000). https://www.quekett.org/resources/understanding/theory-contrast-control. Accessed 21 Jun 2021
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Deka, G., Deka, B., Nishida, K., Fujita, K., Chu, SW. (2022). Deep Tissue High-resolution and Background-free Imaging with Plasmonic SAX Microscopy. In: Biswas, R., Mazumder, N. (eds) Recent Advances in Plasmonic Probes. Lecture Notes in Nanoscale Science and Technology, vol 33. Springer, Cham. https://doi.org/10.1007/978-3-030-99491-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-99491-4_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99490-7
Online ISBN: 978-3-030-99491-4
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)