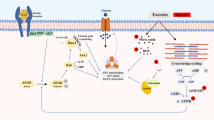
Abstract
Muscle glucose uptake during exercise is regulated by a coordinated increase in glucose delivery (via increased blood flow and glucose moving out of the capillaries into the interstitial space), by facilitated glucose transport into the myocytes and by intramyocellular metabolism. The facilitative glucose transporter GLUT4 is translocated to the sarcolemma and the t-tubules, and GLUT4 translocation is essential for glucose transport into the myocytes during exercise. Several molecular mechanisms have been proposed to regulate insulin-independent GLUT4 translocation during in vivo conditions, but the regulation of both exercise-stimulated GLUT4 translocation and the integrative glucose uptake process by exercise remains incompletely understood. GLUT4 intrinsic transporter activity may also be regulated during exercise although there is no firm evidence for this. The multitude of mechanisms involved in muscle glucose uptake stimulation during exercise ensure the delivery of easily combustible fuel to the working muscles.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abbott MJ, Bogachus LD, Turcotte LP (2011) AMPKα2 deficiency uncovers time dependency in the regulation of contraction-induced palmitate and glucose uptake in mouse muscle. J Appl Physiol 111:125–134
Abel ED et al (1999) Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 104:1703–1714
Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J (1974) Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest 53:1080–1090
Balon TW, Nadler JL (1997) Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol 82:359–363
Bangsbo J, Madsen K, Kiens B, Richter EA (1997) Muscle glycogen synthesis in recovery from intense exercise in humans. Am J Physiol–Endocrinol Metab 273
Birk JB, Wojtaszewski JFP (2006) Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577:1021–1032
Blair DR, Funai K, Schweitzer GG, Cartee GD (2009) A myosin II ATPase inhibitor reduces force production, glucose transport, and phosphorylation of AMPK and TBC1D1 in electrically stimulated rat skeletal muscle. Am J Physiol–Endocrinol Metab 296
Bradley SJ, Kingwell BA, McConell GK (1999) Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 48:1815–1821
Bruton JD, Katz A, Westerblad H (1999) Insulin increases near-membrane but not global Ca2+ in isolated skeletal muscle. Proc Natl Acad Sci U S A 96
Bultot L et al (2016) Benzimidazole derivative small-molecule 991 enhances AMPK activity and glucose uptake induced by AICAR or contraction in skeletal muscle. Am J Physiol–Endocrinol Metab 311:E706–E719
Calbet JAL et al (2007) Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol 103:969–978
Capaldo B et al (1999) Splanchnic and leg substrate exchange after ingestion of a natural mixed meal in humans. Diabetes 48:958–966
Cartee GD, Holloszy JO (1990) Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol–Endocrinol Metab 258
Chambers MA, Moylan JS, Smith JD, Goodyear LJ, Reid MB (2009) Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J Physiol 587:3363–3373
Chen ZP et al (2003) Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52:2205–2212
Christiansen D, Eibye K, Hostrup M, Bangsbo J (2020) Training with blood flow restriction increases femoral artery diameter and thigh oxygen delivery during knee-extensor exercise in recreationally trained men. J Physiol 598:2337–2353
Coyle EF et al (1983) Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J Appl Physiol Respir Environ Exerc Physiol 55:230–235
Dawson D et al (2002) Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol–Endocrinol Metab 282
DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J (1981) Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 68:1468–1474
Dzamko N et al (2008) AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol 586:5819–5831
Erickson JR et al (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133:462–474
Etgen GJ, Fryburg DA, Gibbs EM (1997) Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes 46:1915–1919
Fam BC et al (2012) Normal muscle glucose uptake in mice deficient in muscle GLUT4. J Endocrinol 214:313–327
Fritzen AM et al (2015) 5′-AMP activated protein kinase α2 controls substrate metabolism during post-exercise recovery via regulation of pyruvate dehydrogenase kinase 4. J Physiol 593:4765–4780
Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Wasserman DH (2004a) Distributed control of glucose uptake by working muscles of conscious mice: roles of transport and phosphorylation. Am J Physiol–Endocrinol Metab 286
Fueger PT et al (2004b) Control of exercise-stimulated muscle glucose uptake by GLUT4 is dependent on glucose phosphorylation capacity in the conscious mouse. J Biol Chem 279:50956–50961
Fueger PT et al (2007) Glucose kinetics and exercise tolerance in mice lacking the GLUT4 glucose transporter. J Physiol 582:801–812
Fujii N et al (2005) AMP-activated protein kinase α2 activity is not essential for contraction-and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem 280:39033–39041
Gaster M, Poulsen P, Handberg A, Schrøder HD, Beck-Nielsen H (2000) Direct evidence of fiber type-dependent GLUT-4 expression in human skeletal muscle. Am J Physiol–Endocrinol Metab 278
Gaster M et al (2004) GLUT11, but not GLUT8 or GLUT12, is expressed in human skeletal muscle in a fibre type-specific pattern. Pflugers Arch Eur J Physiol 448:105–113
González-Alonso J, Calbet JAL, Nielsen B (1999a) Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J Physiol 520:577–589
González-Alonso J et al (1999b) Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86:1032–1039
Hargreaves M, Kiens B, Richter EA (1991) Effect of increased plasma free fatty acid concentration on muscle metabolism in exercising men. J Appl Physiol 70:194–201
Havel RJ, Pernow B, Jones NL (1967) Uptake and release of free fatty acids and other metabolites in the legs of exercising men. J Appl Physiol 23:90–99
Heinonen I et al (2013) Effect of nitric oxide synthase inhibition on the exchange of glucose and fatty acids in human skeletal muscle. Nutr Metab (Lond) 10:43
Henríquez-Olguín C et al (2019) The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxid Redox Signal 31:1371–1410
Henríquez-Olguin C et al (2019) Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat Commun 10
Hespel P, Richter EA (1990) Glucose uptake and transport in contracting, perfused rat muscle with different pre-contraction glycogen concentrations. J Physiol 427:347–359
Hespel P, Vergauwen L, Vandenberghe K, Richter EA (1995) Important role of insulin and flow in stimulating glucose uptake in contracting skeletal muscle. Diabetes 44:210–215
Higaki Y, Hirshman MF, Fujii N, Goodyear LJ (2001) Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes 50:241–247
Higaki Y et al (2008) Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am J Physiol–Endocrinol Metab 294
Hingst JR et al (2020) Inducible deletion of skeletal muscle AMPKα reveals that AMPK is required for nucleotide balance but dispensable for muscle glucose uptake and fat oxidation during exercise. Mol. Metab. 40
Hirschfield W et al (2000) Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am J Physiol–Regul Integr Comp Physiol 278
Hong YH, Betik AC, Mcconell GK (2014) Role of nitric oxide in skeletal muscle glucose uptake during exercise. Exp Physiol 99:1569–1573
Hong YH, Yang C, Betik AC, Lee-Young RS, McConell GK (2016) Skeletal muscle glucose uptake during treadmill exercise in neuronal nitric oxide synthase-μ knockout mice. Am J Physiol–Endocrinol Metab 310:E838–E845
Hong YH et al (2015a) No effect of NOS inhibition on skeletal muscle glucose uptake during in situ hindlimb contraction in healthy and diabetic Sprague-Dawley rats. Am J Physiol–Regul Integr Comp Physiol 308:R862–R871
Hong YH et al (2015b) Glucose uptake during contraction in isolated skeletal muscles from neuronal nitric oxide synthase μ knockout mice. J Appl Physiol 118:1113–1121
Howlett KF, Andrikopoulos S, Proietto J, Hargreaves M (2013) Exercise-induced muscle glucose uptake in mice with graded, muscle-specific GLUT-4 deletion. Physiol Rep 1
Huang S, Czech MP (2007) The GLUT4 glucose transporter. Cell Metab 5
Ihlemann J, Ploug T, Galbo H (2001) Effect of force development on contraction induced glucose transport in fast twitch rat muscle. Acta Physiol Scand 171:439–444
Ihlemann J, Ploug T, Hellsten Y, Galbo H (1999) Effect of tension on contraction-induced glucose transport in rat skeletal muscle. Am J Physiol–Endocrinol Metab 277
Ihlemann J, Ploug T, Hellsten Y, Galbo H (2000) Effect of stimulation frequency on contraction-induced glucose transport in rat skeletal muscle. Am J Physiol–Endocrinol Metab 279
Jensen TE, Angin Y, Sylow L, Richter EA (2014) Is contraction-stimulated glucose transport feedforward regulated by Ca2+? Exp Physiol 99
Jensen TE et al (2007) Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol–Endocrinol Metab 292
Jensen TE et al (2012) EMG-normalised kinase activation during exercise is higher in human gastrocnemius compared to soleus muscle. PLoS One 7
Jensen TE et al (2014a) Contraction-stimulated glucose transport in muscle is controlled by AMPK and mechanical stress but not sarcoplasmatic reticulum Ca2+ release. Mol Metab 3
Jensen TE et al (2014c) Contraction-stimulated glucose transport in muscle is controlled by AMPK and mechanical stress but not sarcoplasmic reticulum Ca2+ release. Mol Metab 3
Jørgensen NO et al (2021) Direct small molecule ADaM-site AMPK activators reveal an AMPKγ3-independent mechanism for blood glucose lowering. Mol Metab 51:101259
Joyner MJ, Casey DP (2015) Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol Rev 95:549–601
Kaczmarczyk SJ et al (2003) Threshold effects of glucose transporter-4 (GLUT4) deficiency on cardiac glucose uptake and development of hypertrophy. J Mol Endocrinol 31:449–459
Katz A, Broberg S, Sahlin K, Wahren J (1986a) Muscle ammonia and amino acid metabolism during dynamic exercise in man. Clin Physiol 6:365–379
Katz A, Broberg S, Sahlin K, Wahren J (1986b) Leg glucose uptake during maximal dynamic exercise in humans. Am J Physiol–Endocrinol Metab 251
Katz A, Sahlin K, Broberg S (1991) Regulation of glucose utilization in human skeletal muscle during moderate dynamic exercise. Am J Physiol–Endocrinol Metab 260
Katz EB, Stenbit AE, Hatton K, Depinho R, Charron MJ (1995) Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377:151–155
Kemppainen J et al (2002) Myocardial and skeletal muscle glucose uptake during exercise in humans. J Physiol 542:403–412
Kennedy JW et al (1999) Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 48:1192–1197
Khayat ZA, Tong P, Yaworsky K, Bloch RJ, Klip A (2000) Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J Cell Sci 113:279–290
Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK (2002) Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes 51:2572–2580
Kjaer M, Kiens B, Hargreaves M, Richter EA (1991) Influence of active muscle mass on glucose homeostasis during exercise in humans. J Appl Physiol 71:552–557
Kjøbsted R et al (2018) AMPK in skeletal muscle function and metabolism. FASEB J 32:1741–1777
Kjøbsted R et al (2019) AMPK and TBC1D1 regulate muscle glucose uptake after, but not during, exercise and contraction. Diabetes 68:1427–1440
Kleinert M et al (2017) Mammalian target of rapamycin complex 2 regulates muscle glucose uptake during exercise in mice. J Physiol 595:4845–4855
Knudsen JR et al (2020) Growth factor-dependent and -independent activation of mTORC2. Trends Endocrinol Metab 31:13–24
Kristiansen S, Hargreaves M, Richter EA (1996a) Exercise-induced increase in glucose transport, GLUT-4, and VAMP-2 in plasma membrane from human muscle. Am J Physiol–Endocrinol Metab 70(1):E197–E201
Kristiansen S, Youn J, Richter EA (1996b) Effect of vanadate on glucose transporter (GLUT4) intrinsic activity in skeletal muscle plasma membrane giant vesicles. Biochim Biophys Acta 1282:71–75
Kristiansen S, Asp S, Richter EA (1996c) Decreased muscle GLUT-4 and contraction-induced glucose transport after eccentric contractions. Am J Physiol–Regul Integr Comp Physiol 271
Kristiansen S, Hargreaves M, Richter EA (1997) Progressive increase in glucose transport and GLUT-4 in human sarcolemmal vesicles during moderate exercise. Am J Physiol–Endocrinol Metab 272
Lang F, Artunc F, Vallon V (2009) The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr Opin Nephrol Hypertens 18:439–448
Lanner JT et al (2006) The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes 55:2077–2083
Lanner JT et al (2009) Knockdown of TRPC3 with siRNA coupled to carbon nanotubes results in decreased insulin-mediated glucose uptake in adult skeletal muscle cells. FASEB J 23:1728–1738
Lau KS et al (2000) nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2000:21–27
Lawrence JC, Piper RC, Robinson LJ, James DE (1992) GLUT4 facilitates insulin stimulation and cAMP-mediated inhibition of glucose transport. Proc Natl Acad Sci U S A 89:3493–3497
Lee-Young RS et al (2009) Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem 284:23925–23934
Lee-Young RS et al (2010) Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am J Physiol–Regul Integr Comp Physiol 298
Linden KC, Wadley GD, Garnham AP, McConell GK (2011) Effect of l-arginine infusion on glucose disposal during exercise in humans. Med Sci Sports Exerc 43:1626–1634
Luczak ED, Anderson ME (2014) CaMKII oxidative activation and the pathogenesis of cardiac disease. J Mol Cell Cardiol 73:112–116
Lund S, Holman GD, Schmitz O, Pedersen O (1995) Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A 92:5817–5821
Lundsgaard AM, Kiens B (2014) Gender differences in skeletal muscle substrate metabolism–molecular mechanisms and insulin sensitivity. Front Endocrinol 5
Maarbjerg SJ et al (2009) Genetic impairment of AMPKα2 signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am J Physiol–Endocrinol Metab 297
MacLean DA, Bangsbo J, Saltin B (1999) Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. J Appl Physiol 87:1483–1490
McConell GK (2020) It’s well and truly time to stop stating that AMPK regulates glucose uptake and fat oxidation during exercise. Am J Physiol Endocrinol Metab 318:E564–E567
McConell G, Fabris S, Proietto J, Hargreaves M (1994) Effect of carbohydrate ingestion on glucose kinetics during exercise. J Appl Physiol 77:1537–1541
McConell GK, Wadley GD, Le Plastrier K, Linden KC (2020) Skeletal muscle AMPK is not activated during 2 h of moderate intensity exercise at ∼65% V̇O2peak in endurance trained men. J Physiol 598:3859–3870
McConell GK et al (2005) Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol 568:665–676
McMillin SL, Schmidt DL, Kahn BB, Witczak CA (2017) GLUT4 is not necessary for overload-induced glucose uptake or hypertrophic growth in mouse skeletal muscle. Diabetes 66:1491–1500. (American Diabetes Association Inc.
Merry TL, Dywer RM, Bradley EA, Rattigan S, McConell GK (2010a) Local hindlimb antioxidant infusion does not affect muscle glucose uptake during in situ contractions in rat. J Appl Physiol 108:1275–1283
Merry TL, Steinberg GR, Lynch GS, McConell GK (2010b) Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol–Endocrinol Metab 298
Merry TL, Lynch GS, McConell GK (2010c) Downstream mechanisms of nitric oxide-mediated skeletal muscle glucose uptake during contraction. Am J Physiol–Regul Integr Comp Physiol 299
Merry TL et al (2010d) N-acetylcysteine infusion does not affect glucose disposal during prolonged moderate-intensity exercise in humans. J Physiol 588:1623–1634
Møller LLV et al (2020) The p21-activated kinase 2 (PAK2), but not PAK1, regulates contraction-stimulated skeletal muscle glucose transport. Physiol Rep 8
Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y (2012) The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590:4391–4400
Mortensen SP et al (2005) Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol 566:273–285
Mortensen SP et al (2009) ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol–Regul Integr Comp Physiol 296
Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094
Pappenheimer JR, Renkin EM, Borrero LM (1951) Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Phys 167:13–46
Park DR, Park KH, Kim BJ, Yoon CS, Kim UH (2015) Exercise ameliorates insulin resistance via Ca2+ signals distinct from those of insulin for GLUT4 translocation in skeletal muscles. Diabetes 64:1224–1234
Patwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ (2004) Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37:1064–1072
Ploug T et al (1998) Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol 142. http://www.jcb.org
Raun SH et al (2018) Rac1 muscle knockout exacerbates the detrimental effect of high-fat diet on insulin-stimulated muscle glucose uptake independently of Akt. J Physiol 596
Reichard GA et al (1961) Blood glucose metabolism in man during muscular work. J Appl Physiol 16:1001–1005
Rhein P et al (2021) Compound- and fiber type-selective requirement of AMPKγ3 for insulin-independent glucose uptake in skeletal muscle. Mol Metab 51:101228
Richter EA (1996) Glucose utilization. Handb Physiol Sect 12 Exerc Regul Integr Mult Syst Ed by Rowell LB Shepherd JT New York Oxford Univ Press
Richter EA (2020) Is GLUT4 translocation the answer to exercise stimulated muscle glucose uptake? Am J Physiol Endocrinol Metab 320(2):E240–E243
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017
Roberts CK, Barnard RJ, Scheck SH, Balon TW (1997) Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol–Endocrinol Metab 273
Roepstorff C et al (2002) Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol–Endocrinol Metab 282
Roepstorff C et al (2005) Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol–Endocrinol Metab 288
Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P (2018) Endothelial cell metabolism in health and disease. Trends Cell Biol 28:224–236
Romijn JA et al (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab 265
Rose AJ, Alsted TJ, Kobberø JB, Richter EA (2007) Regulation and function of Ca2+−calmodulin-dependent protein kinase II of fast-twitch rat skeletal muscle. J Physiol 580:993–1005
Rose AJ, Kiens B, Richter EA (2006) Ca2+−calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol 574:889–903
Ross RM, Wadley GD, Clark MG, Rattigan S, McConell GK (2007) Local nitric oxide synthase inhibition reduces skeletal muscle glucose uptake but not capillary blood flow during in situ muscle contraction in rats. Diabetes 56:2885–2892
Ryder JW et al (1999a) In vitro analysis of the glucose-transport system in GLUT4-null skeletal muscle. Biochem J 342:321–328
Ryder JW et al (1999b) Postexercise glucose uptake and glycogen synthesis in skeletal muscle from GLUT4-deficient mice. FASEB J 13:2246–2256
Sakamoto K, Aschenbach WG, Hirshman MF, Goodyear LJ (2003) Akt signaling in skeletal muscle: regulation by exercise and passive stretch. Am J Physiol–Endocrinol Metab 285
Sanders CA, Levinson GE, Abelmann WH, Freinkel N (1964) Effect of exercise on the peripheral utilization of glucose in man. N Engl J Med 271:220–225
Sandström ME et al (2006) Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol 575:251–262
Schultz TA, Lewis SB, Westbie DK (1977) Glucose delivery: a modulator of glucose uptake in contracting skeletal muscle. Am J Physiol Endocrinol Metab Gastrointest Physiol 2
Shamni O et al (2017) Supportive data on the regulation of GLUT4 activity by 3-O-methyl-D-glucose. Data Br 14:329–336
Silveira LR, Pereira-Da-Silva L, Juel C, Hellsten Y (2003) Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med 35:455–464
Sjøberg KA, Rattigan S, Hiscock N, Richter EA, Kiens B (2011) A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. Am J Physiol Heart Circ Physiol 301
Sjøberg KA et al (2017) Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66:1501–1510
Stenbit AE et al (1996) Diverse effects of Glut 4 ablation on glucose uptake and glycogen synthesis in red and white skeletal muscle. J Clin Invest 98:629–634
Sylow L, Kleinert M, Richter EA, Jensen TE (2017a) Exercise-stimulated glucose uptake-regulation and implications for glycaemic control. Nat Rev Endocrinol 13:133–148
Sylow L, Møller LLV, Kleinert M, Richter EA, Jensen TE (2014) Rac1–a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Exp Physiol 99:1574–1580
Sylow L, Møller LLV, Kleinert M, Richter EA, Jensen TE (2015) Stretch-stimulated glucose transport in skeletal muscle is regulated by Rac1. J Physiol 593
Sylow L et al (2013a) Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes 62:1139–1151
Sylow L et al (2013b) Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes 62(6):1865–1875
Sylow L et al (2016) Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J Physiol 594:4997–5008
Sylow L et al (2017b) Rac1 and AMPK account for the majority of muscle glucose uptake stimulated by ex vivo contraction but not in vivo exercise. Diabetes 66
Treebak JT et al (2007) AS160 phosphorylation is associated with activation of α 2β2γ1- but not α 2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol–Endocrinol Metab 292
Ueda S et al (2010) Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J 24(7):2254–2261
Van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM (2001) The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536:295–304
Vassilopoulos S et al (2009) A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science (80-) 324:1192–1196
Wahren J, Felig P, Ahlborg G, Jorfeldt L (1971) Glucose metabolism during leg exercise in man. J Clin Invest 50:2715–2725
Wang W, Hansen PA, Marshall BA, Holloszy JO, Mueckler M (1996) Insulin unmasks a COOH-terminal GLUT4 epitope and increases glucose transport across T-tubules in skeletal muscle. J Cell Biol 135:415–430
Wang Q et al (2018) Oxidized CaMKII (ca 2+ /calmodulin-dependent protein kinase II) is essential for ventricular arrhythmia in a mouse model of Duchenne muscular dystrophy. Circ Arrhythmia Electrophysiol 11
Whichelow MJ, Butterfield WJH, Abrams ME, Sterky G, Garratt CJ (1968) The effect of mild exercise on glucose uptake in human forearam tissues in the fasting state and after oral glucose administration. Metabolism 17:84–96
Witczak CA et al (2010) CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am J Physiol–Endocrinol Metab 298
Wojtaszewski JFP, Nielsen P, Hansen BF, Richter EA, Kiens B (2000) Isoform-specific and exercise intensity-dependent activation of 5’-AMP-activated protein kinase in human skeletal muscle. J Physiol 528:221–226
Wright DC, Hucker KA, Holloszy JO, Han DH (2004) Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 53:330–335
Yazdani S, Jaldin-Fincati JR, Pereira RVS, Klip A (2019) Endothelial cell barriers: transport of molecules between blood and tissues. Traffic 20:390–403
Zaid H, Talior-Volodarsky I, Antonescu C, Liu Z, Klip A (2009) GAPDH binds GLUT4 reciprocally to hexokinase-II and regulates glucose transport activity. Biochem J 419:475–484
Zierath JR et al (1998) Restoration of hypoxia-stimulated glucose uptake in GLUT4-deficient muscles by muscle-specific GLUT4 transgenic complementation. J Biol Chem 273:20910–20915
Zinker BA, Lacy DB, Bracy DP, Wasserman DH (1993) Role of glucose and insulin loads to the exercising limb in increasing glucose uptake and metabolism. J Appl Physiol 74:2915–2921
Zisman A et al (2000) Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6:924–928
Funding
This work was supported by grants from the Novo Nordisk foundation, the Independent Research Fund Denmark, and/or the Lundbeck foundation to LSH, TEJ, GKM, and EAR and the Danish Diabetes Academy funded by the Novo Nordisk Foundation to COL, JRK, and GKM.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jensen, T.E., Knudsen, J.R., Henriquez-Olguin, C., Sylow, L., McConell, G., Richter, E.A. (2022). Exercise-Regulated Skeletal Muscle Glucose Uptake. In: McConell, G. (eds) Exercise Metabolism. Physiology in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-94305-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-94305-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94304-2
Online ISBN: 978-3-030-94305-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)