Abstract
Certain medical devices utilize materials of animal origin. Animal tissues and their derivatives are used in the design and manufacture of medical devices to provide performance characteristics that have been chosen for advantages over nonanimal-based materials. The range and quantities of materials of animal origin in medical devices vary. These materials can comprise a major part of the device (e.g. bovine/porcine heart valves, bone substitutes for use in dental or orthopaedic applications, haemostatic devices), can be a product coating or impregnation (e.g. collagen, gelatine, heparin) or can be used in the device manufacturing process (e.g. tallow derivatives such as oleates and stearates, foetal calf serum, enzymes, culture media). This part of ISO 22442 provides additional requirements and guidance for the evaluation of medical devices manufactured utilizing animal tissues or derivatives which are non-viable or rendered non-viable. This part of ISO 22442 is intended to cover medical devices including active implantable medical devices such as implantable infusion pumps. ISO 22442 consists of the following parts, under the general title Medical devices utilizing animal tissues and their derivatives:
-
Part 1: Application of risk management
-
Part 2: Controls on sourcing, collection and handling
-
Part 3: Validation of the elimination and/or inactivation of viruses and transmissible spongiform encephalopathy (TSE) agent
-
Part 4: Principles for elimination and/or inactivation of transmissible spongiform encephalopathy (TSE) agents and validation assays for those processes
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Abbreviations
- AAMI:
-
Association for the Advancement of Medical Instrumentation
- BSE:
-
Bovine spongiform encephalopathy
- CDC:
-
Centers for Disease Control and Prevention
- CJD:
-
Creutzfeldt-Jakob disease
- CPMP:
-
Certified Project Management Professional
- GMP:
-
Good manufacturing processes
- ISO:
-
International Organization for Standardization
- NaOH:
-
Sodium hydroxide
- PrPTSE:
-
Prion protein associated transmissible spongiform encephalopathy
- PRNP:
-
Prion protein
- TSE:
-
Transmissible spongiform encephalopathy
- WHO:
-
World Health Organization
- OIE:
-
World Organisation for Animal Health
References
BS EN ISO 22442-1:2015 Medical devices utilizing animal tissues and their derivatives Part 1: Application of risk management
BS EN ISO 22442-2:2015 Medical devices utilizing animal tissues and their derivatives Part 2: Controls on sourcing, collection and handling
BS EN ISO 22442-3:2007 Medical devices utilizing animal tissues and their derivatives Part 3: Validation of the elimination and/or inactivation of viruses and transmissible spongiform encephalopathy (TSE) agents
ISO/TR 22442-4: Medical devices utilizing animal tissues and their derivatives — Part 4: Principles for elimination and/or inactivation of transmissible spongiform encephalopathy (TSE) agents and validation assays for those processes
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Annexures
Annexures
1.1 Annexure A: Guidance on the Application of This Part of ISO 22442
Application to Materials from Animal Sources
This international standard is applicable to materials such as:
-
Porcine heart valves, bovine bones, cattle ligaments and bovine pericardium
-
Derivatives of animal tissues, such as chondroitin sulphate obtained from shark and collagen derived from hides, and of animal blood or serum
-
Materials produced in vivo by relevant animals, e.g. antibodies utilized in the manufacturing process
-
Starting materials such as bovine serum albumin, enzymes, culture media including those used to prepare working cell banks, master cell banks or master seeds for products such as hyaluronic acid
Application to Materials Supplied by Third Parties
This part of ISO 22442 can be applied when the materials used by medical device manufacturers have been prepared from animal sources by third parties or subcontractors. An example is gelatine derived from animal hides or bones. In considering the risks associated with the use of these products, the medical device manufacturers should seek evidence from their suppliers as to whether relevant requirements of this international standard have been applied in assessing the suitability of the animal material or whether alternative approaches were applied.
1.2 Annexure B: Graphical Representation of Part of the Risk Management Process for Medical Devices Utilizing Animal Material
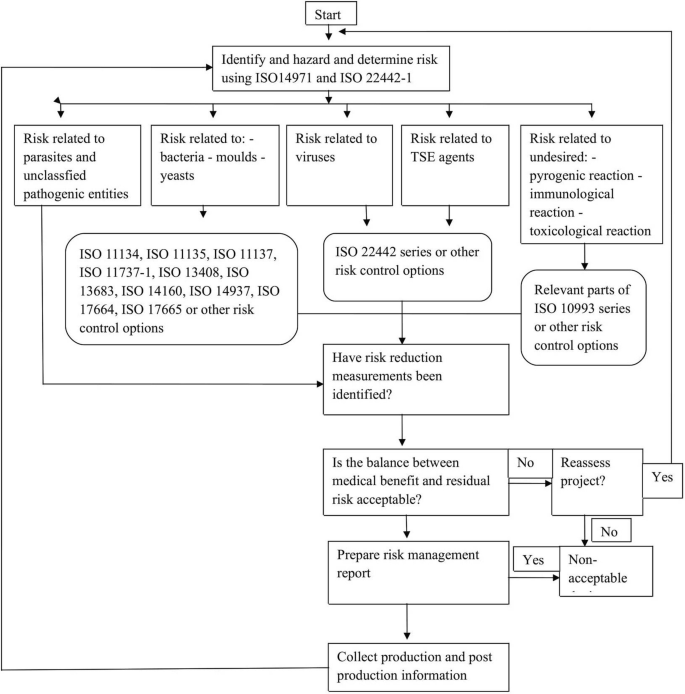
1.3 Annexure C: Special Requirements for Some Animal Materials Considering the Risk Management for TSE Agents
C.1 Collagen
Collagen is a fibrous protein component of mammalian connective tissue. For collagen, documentation to demonstrate compliance with this part of ISO 22442 shall be provided, taking into account the relevant requirements of this annex. When completing the risk management required by this part of ISO 22442, consider the following: For collagen produced from bone, the bone shall be sourced from countries with minimal exposure to BSE. Sourcing bone from countries with limited exposure to BSE shall be justified by reference to other applicable risk control measures. Bone shall not be sourced from countries where infection with the BSE agent is confirmed at a higher level, unless from a low-risk herd as defined in ISO 22442-2. For collagen produced from bones, the manufacturing conditions specified for gelatine are applicable.
-
Collagen produced from hides and skins does not usually present a significant TSE risk provided that cross-contamination with potentially infected materials, for example, central nervous tissues, is avoided during their procurement. To demonstrate compliance with the requirements of this part of ISO 22442, it is necessary to incorporate measures to prevent cross-contamination and to document the measures that are adopted in the technical agreement between the collagen supplier and the medical device manufacturer to prevent such cross-contamination. Collagen shall be obtained from animals declared as fit for human consumption.
C.2 Gelatine Derived from Hides and Bones
Gelatine is a natural, soluble protein, gelling or non-gelling, obtained by the partial hydrolysis of collagen produced from bones, hides and skins, tendons and sinews of animals. For gelatine, documentation to demonstrate compliance with this part of ISO 22442 shall be provided, taking into account the relevant requirements listed in this annex. Gelatine shall be obtained from animals declared as fit for human consumption.
Hides as the Starting Material
On the basis of current knowledge, hides used for gelatine production represent a safer source material when compared to bones. Gelatine produced from hides does not usually present a significant TSE risk provided that cross-contamination with potentially infected materials, for example, central nervous tissues, is avoided during their procurement. To demonstrate compliance with the requirements of this part of ISO 22442, it is necessary to incorporate measures to prevent cross-contamination and to document the measures that are adopted to prevent such cross-contamination in the technical agreement between the gelatine supplier and the medical device manufacturer.
Bones as the Starting Material
Where bones are used to manufacture gelatine, the quality of the starting materials is the primary parameter that will ensure the safety of the final product. Therefore, the following shall be applied:
-
Subject to national legislation, bone shall be sourced from countries with minimal or limited exposure to BSE. Bone shall not be sourced from countries where infection with the BSE agent is confirmed at a higher level, unless from a low-risk herd as defined in ISO 22442-2.
-
Skulls and spinal cords shall be removed from the collected bones (raw/starting material) from cattle of a specific age as defined in national legislation.
-
Additionally, vertebrae shall be removed from the raw/starting materials from cattle of all ages from countries with limited exposure to BSE.
C.3 Bovine Blood Derivatives
General foetal bovine serum is commonly used in cell cultures. Foetal bovine serum should be obtained from foetuses harvested in abattoirs from healthy dams fit for human consumption, and the womb should be completely removed. The foetal blood shall be harvested in a dedicated space or area by cardiac puncture into a closed collection system using an aseptic technique. New born calf serum is obtained from calves aged less than 20 days and calf serum from animals aged less than 12 months. In the case of donor bovine serum, given that it can be derived from animals less than 36 months old, the BSE status of the donor herd shall be well defined and documented. In all cases, serum shall be collected according to specified protocols by personnel trained in these procedures and the precautions necessary to avoid cross-contamination with higher-risk tissues.
C.4 Stunning Methods
If blood is obtained from slaughtered animals, the method of slaughter is of importance to ensure the safety of the material. It has been demonstrated that stunning by a captive bolt stunner with or without pithing, as well as by pneumatic stunner, especially if it injects air, can destroy the brain and disseminate brain material into the blood stream. There is evidence that non-penetrative stunning can cause some central nervous system (CNS) embolism. The stunning methods shall be described for the bovine blood collection process unless the material is sourced from a country of negligible geographical BSE risk. Where sourcing of blood is from countries with limited exposure to BSE, a non-penetrative stunner or electronarcosis shall be used for slaughter of animals over 12 months of age. The use of non-penetrative stunning shall be justified on the basis of an estimate of the risk of dissemination of brain particles into the blood.
C.5 Tallow Derivatives
Tallow is fat obtained from tissues including subcutaneous, abdominal and intermuscular areas and bones. Tallow derivatives, such as glycerol and fatty acids, manufactured from tallow by rigorous processes, are thought unlikely to be infectious. For this reason, such materials manufactured under the conditions at least as rigorous as those given below shall be considered as presenting an acceptable TSE risk, irrespective of the geographical origin and the nature of the tissues from which tallow derivatives are derived.
C.6 Animal Charcoal
Animal charcoal is prepared by carbonization of animal tissues, such as bones, using a temperature >800 °C. Irrespective of the geographical origin and the nature of the tissue, animal charcoal prepared under these conditions shall be considered as presenting an acceptable TSE risk.
C.7 Milk and Milk Derivatives
Certain materials, including lactose, are extracted from whey, the spent liquid from cheese production following coagulation. Coagulation can involve the use of calf rennet, an extract from abomasums, or rennet derived from other ruminants. A risk assessment for lactose and other whey derivatives produced using calf rennet was performed and concluded that the TSE risk is negligible if the calf rennet is produced in accordance with the process described in the CPMP risk assessment report. Subject to national legislation, milk derivatives manufactured according to the conditions below are considered as presenting an acceptable TSE risk: the milk is sourced from healthy animals under the same conditions as milk collected for human consumption; no other ruminant-derived materials, with the exception of calf rennet, are used in the preparation of such derivatives (e.g. pancreatic enzyme digests of casein).
C.8 Wool and Its Derivatives
Wool and its derivatives, such as lanolin and wool alcohols, shall be considered in compliance with this part of ISO 22442, provided the wool is sourced from live healthy animals. Wool derivatives produced from wool that is sourced from slaughtered animals declared “fit for human consumption” are considered as presenting an acceptable TSE risk if the manufacturing process in relation to pH, temperature and duration of treatment meets at least one of the stipulated processing conditions listed as follows: treatment at pH ≥13 (initial; corresponding to concentrations of sodium hydroxide ≥0,1 mol/l) at ≥60 °C for at least 1 h; this normally occurs during the reflux stage of the organic-alkaline treatment; molecular distillation is at ≥220 °C under reduced pressure.
C.9 Amino Acids
Amino acids can be obtained by hydrolysis of animal materials from various sources. Amino acids prepared using the following processing conditions are considered as presenting an acceptable TSE risk: amino acids produced from hides and skins by a process which involves exposure of the material to a pH of 1–2, followed by a pH >11 and heat treatment at 140 °C for 30 min at 3 bar; the resulting amino acids or peptides shall be filtered after production; analysis shall be performed using a validated and sensitive method to control any residual intact macromolecules with a justified limit set.
1.4 Annexure D: Information Relevant to the Management of TSE Risk
TSE
The naturally occurring transmissible spongiform encephalopathies (TSEs) include scrapie (in sheep and goats), chronic wasting disease (in mule deer and elk), bovine spongiform encephalopathy (BSE) in cattle as well as kuru and Creutzfeldt-Jakob disease (CJD) in humans. It is difficult to detect agents causing these diseases in vivo. After latency periods of up to many years, the agents cause disease and, finally, lead to death. No means of therapy is known. Current information on the characteristics of the causative agents is limited. These agents are extremely resistant to most of the chemical and physical procedures that inactivate conventional viruses. They do not induce a detectable immune response. There are natural barriers which limit their interspecies spread of transmissible agent, but they can be crossed under appropriate circumstances. This is usually dependent upon strain, dose, route of exposure and the species barrier. Studies in laboratory animals have shown that intracerebral inoculation is the most efficient route of transmission.
Risks for Humans
There is considerable circumstantial evidence that the variant form of human CJD (vCJD) arose from BSE and it is prudent to accept that the BSE agent can be transmitted to man. This part of ISO 22442 therefore contains a number of requirements to ensure that risks are controlled if biological materials from species susceptible to TSE are used for the manufacture of medical devices. This annex provides guidance that should be followed to minimize the risks of contamination. It identifies where requirements elsewhere in this part of ISO 22442 are applicable and where information from other sources is relevant. All devices should be considered on a case-by-case basis.
Risk Management for TSE Agents
The safety of a medical device, in terms of its potential for passing on a TSE agent, is dependent on a number of factors. The eight most important factors below should be analysed, evaluated and managed:
-
Animal species used
-
Geographical sourcing
-
Nature of starting tissue
-
Slaughtering and processing controls to prevent cross-contamination
-
Methods used to inactivate or remove TSE agents
-
Quantities of animal starting material required to produce one unit of the medical device
-
Quantities of material of animal origin coming into contact with the patients and users
-
Route of administration
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sampath, T., Thamizharasan, S., V., K., Timiri Shanmugam, P.S. (2022). ISO 22442: Medical Devices Utilizing Animal Tissues and Their Derivatives. In: Timiri Shanmugam, P.S., Thangaraju, P., Palani, N., Sampath, T. (eds) Medical Device Guidelines and Regulations Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-91855-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-91855-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91854-5
Online ISBN: 978-3-030-91855-2
eBook Packages: EngineeringEngineering (R0)

