Abstract
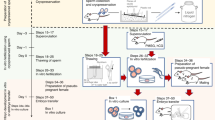
New genome-editing tools, such as ZFNs, TALEN, and CRISPR/Cas9, have enabled the generation of gene-modified models effectively in mammals. These technologies are a powerful tool for studying gene function and creating animal models for human diseases. On the other hand, such gene-modified animals are raised in numerous experimental animal facilities, which puts pressure on breeding space and maintenance costs. Embryo and sperm cryopreservation is not only the most simple and cost-effective method available for most gene-modified strains but also the most reliable method to preserve strains to avoid breeding problems and contamination. We have established a reliable, high quality embryo and sperm cryopreservation system for rat strains, ensuring the longevity of these valuable resources for the scientific community. These cryopreserved resources have been successfully used to rederive next generation pups using embryo transfer and intracytoplasmic sperm injection (ICSI). In this chapter, we describe in detail protocols for rat embryo vitrification and sperm cryopreservation followed by pup rederivation using the ICSI procedure and embryo transfer.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Mashimo T, Yanagihara K, Tokuda S, Voigt B, Takizawa A, Nakajima R et al (2008) An ENU-induced mutant archive for gene targeting in rats. Nat Genet 40:514–515
Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM et al (2009) Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325:433
Mashimo T, Kaneko T, Sakuma T, Kobayashi J, Kunihiro Y, Voigt B et al (2013) Efficient gene targeting by TAL effector nucleases coinjected with exonucleases in zygotes. Sci Rep 3:1253
Li D, Qiu Z, Shao Y, Chen Y, Guan Y, Liu M et al (2013) Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat Biotechnol 31:681–683
Festing MFW (1974) Embryo banks in the production of genetically defined laboratory animals: a step towards the concept of type culture collection of defined laboratory animals. In: Zeilmarker GH (ed) Frozen storage of laboratory animals. Gustav Fischer Verlag, Stuttgart, New York, pp 149–155
Whittingham DG (1975) Survival of rat embryos after freezing and thawing. J Reprod Fertil 43:575–578
Hirabayashi M, Takahashi R, Sekiguchi J, Ueda M (1997) Viability of transgenic rat embryos after freezing and thawing. Exp Anim 46:111–115
Kono T, Suzuki O, Tsunoda Y (1988) Cryopreservation of rat blastocysts by vitrification. Cryobiology 25:170–173
Takahashi R, Hirabayashi M, Ueda M (1990) Production of transgenic rats using cryopreserved pronuclear-stage zygotes. Transgenic Res 8:397–400
Han MS, Niwa K, Kasai M (2003) Vitrification of rat embryos at various developmental stages. Theriogenology 59:1851–1863
Eto T, Takahashi R, Kamisako T, Hioki K, Sotomaru Y (2014) A study on cryoprotectant solution suitable for vitrification of rat two-cell stage embryos. Cryobiology 68:147–151
Nakatsukasa E, Inomata T, Ikeda T, Shino M, Kashiwazaki N (2001) Generation of live rat offspring by intrauterine insemination with epididymal spermatozoa cryopreserved at −196 degrees C. Reproduction 122:463–467
Nakatsukasa E, Kashiwazaki N, Takizawa A, Shino M, Kitada K, Serikawa T et al (2003) Cryopreservation of spermatozoa from closed colonies, and inbred, spontaneous mutant, and transgenic strains of rats. Comp Med 53:639–641
Seita Y, Sugio S, Ito J, Kashiwazaki N (2009) Generation of live rats produced by in vitro fertilization using cryopreserved spermatozoa. Biol Reprod 80:503–510
Seita Y, Fujiwara K, Takizawa A, Furukawa K, Inomata T, Ito J et al (2011) Full-term development of rats from oocytes fertilized in vitro using cryopreserved ejaculated sperm. Cryobiology 63:7–11
Kimura Y, Yanagimachi R (1995) Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development 121:2397–2405
Hirabayashi M, Kato M, Aoto T, Sekimoto A, Ueda M, Miyoshi I et al (2002) Offspring derived from intracytoplasmic injection of transgenic rat sperm. Transgenic Res 11:221–228
Perry ACF, Wakayama T, Kishikawa H, Kasai T, Okabe M, Toyoda Y et al (1999) Mammalian transgenesis by intracytoplasmic sperm injection. Science 284:1180–1183
Kato M, Ishikawa A, Kaneko R, Yagi T, Hochi S, Hirabayashi M (2004) Production of transgenic rats by ooplasmic injection of spermatogenic cells exposed to exogenous DNA: a preliminary study. Mol Reprod Dev 69:153–158
Whittingham DG (1974) Embryo banks in the future of developmental genetics. Genetics 78:395–402
Toyoda Y, Chang MC (1974) Fertilization of rat eggs in vitro by epididymal spermatozoa and the development of eggs following transfer. J Reprod Fertil 36:9–22
Mukumoto S, Mori K, Ishikawa H (1995) Efficient induction of superovulation in adult rats by PMSG and hGH. Exp Anim 44:111–118
Hirabayashi M, Ito K, Sekimoto A, Hochi S, Ueda M (2001) Production of transgenic rats using young Sprague-Dawley females treated with PMSG and hCG. Exp Anim 50:365–369
Popova E, Krivokharchenko A, Ganten D, Bader M (2002) Comparison between PMSG- and FSH-induced superovulation for the generation of transgenic rats. Mol Reprod Dev 63:177–182
Corbin TJ, McCabe JG (2002) Strain variation of immature female rats in response to various superovulatory hormone preparations and routes of administration. Contemp Top Lab Anim Sci 41:18–23
Zernicka-Goetz M (1991) Spontaneous and induced activation of rat oocytes. Mol Reprod Dev 28:169–176
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R24HL114474).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Takizawa, A., Eto, T. (2019). Protocols for Cryopreservation and Rederivation of Rat Gametes. In: Hayman, G., Smith, J., Dwinell, M., Shimoyama, M. (eds) Rat Genomics. Methods in Molecular Biology, vol 2018. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9581-3_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9581-3_6
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-9580-6
Online ISBN: 978-1-4939-9581-3
eBook Packages: Springer Protocols