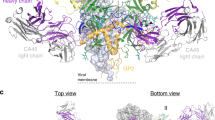
Abstract
Ebola (EBOV) and Marburg (MARV) viruses cause hemorrhagic fever disease in humans and non-human primates (NHPs) with case-fatality rates as high as 90%. The 2013–2016 Ebola virus disease (EVD) outbreak led to over 28,000 cases and 11,000 deaths and took an enormous toll on the economy of West African nations, in the absence of any vaccine or therapeutic options. Like EVD, there have been at least 6 outbreaks of MVD with ~88% case-fatality and the most recent cases emerging in Equatorial Guinea in February 2023. These outbreaks have spurred an unprecedented global effort to develop vaccines and therapeutics for EVD and MVD and led to an approved vaccine (ERVEBO™) and two monoclonal antibody (mAb) therapeutics for EBOV. In contrast to EVD, therapeutic options against Marburg and another Ebola-relative Sudan virus (SUDV) are lacking. The filovirus glycoprotein (GP), which mediates host cell entry and fusion, is the primary target of neutralizing antibodies. In addition to its pre- and post-fusion trimeric states, the protein is highly glycosylated making production of pure and homogeneous trimers on a large scale, a requirement for subunit vaccine development, a challenge. In efforts to address this roadblock, we have developed a unique combination of structure-based design, selection of expression system, and purification methods to produce uniform and stable EBOV and MARV GP trimers at scales appropriate for vaccine production.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Beer B, Kurth R, Bukreyev A et al (1999) Characteristics of filoviridae: marburg and ebola viruses. Naturwissenschaften 86(1):8–17
Hensley LE, Alves DA, Geisbert JB et al (2011) Pathogenesis of Marburg hemorrhagic fever in cynomolgus macaques. J Infect Dis 204(Suppl 3):S1021–S1031
Towner JS, Sealy TK, Khristova ML et al (2008) Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog 4(11):e1000212
Brauburger K, Hume AJ, Mühlberger E et al (2012) Forty-five years of Marburg virus research. Viruses 4(10):1878–1927
Saeed MF, Kolokoltsov AA, Albrecht T et al (2010) Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog 6(9):e1001110
Hashiguchi T, Fusco ML, Bornholdt ZA et al (2015) Structural basis for Marburg virus neutralization by a cross-reactive human antibody. Cell 160(5):904–912
Howell KA, Qiu X, Brannan JM et al (2016) Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep 15(7):1514–1526
King LB, Fusco ML, Flyak AI et al (2018) The marburgvirus-neutralizing human monoclonal antibody MR191 targets a conserved site to block virus receptor binding. Cell Host Microbe 23(1):101–109
Wang Y, Howell KA, Brannan J et al (2021) Prominent neutralizing antibody response targeting the ebolavirus glycoprotein subunit interface elicited by immunization. J Virol 95(8):e01907–e01920
Fusco ML, Hashiguchi T, Cassan R et al (2015) Protective mAbs and cross-reactive mAbs raised by immunization with engineered Marburg virus GPs. PLoS Pathog 11(6):e1005016
Holtsberg FW, Shulenin S, Vu H (2016) Pan-ebolavirus and pan-filovirus mouse monoclonal antibodies: protection against Ebola and Sudan viruses. J Virol 90(1):266–278
Keck ZY, Enterlein SG, Howell KA et al (2016) Macaque monoclonal antibodies targeting novel conserved epitopes within filovirus glycoprotein. J Virol 90(1):279–291
West BR, Wec AZ, Moyer CL et al (2019) Structural basis of broad ebolavirus neutralization by a human survivor antibody. Nat Struct Mol Biol 26(3):204–212
Bornholdt ZA, Ndungo E, Fusco ML et al (2016) Host-primed Ebola virus GP exposes a hydrophobic NPC1 receptor-binding pocket, revealing a target for broadly neutralizing antibodies. MBio 7(1):e02154–e02115
Lee JE, Fusco ML, Hessell AJ et al (2008) Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 454(7201):177–182
Bale S, Dias JM, Fusco ML et al (2012) Structural basis for differential neutralization of ebolaviruses. Viruses 4(4):447–470
Jeffers SA, Sanders DA, Sanchez A (2002) Covalent modifications of the ebola virus glycoprotein. J Virol 76(24):12463–12472
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Noonan-Shueh, M., Aman, M.J., Kailasan, S. (2024). Production and Purification of Filovirus Glycoproteins. In: Bradfute, S.B. (eds) Recombinant Glycoproteins. Methods in Molecular Biology, vol 2762. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3666-4_2
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3666-4_2
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3665-7
Online ISBN: 978-1-0716-3666-4
eBook Packages: Springer Protocols