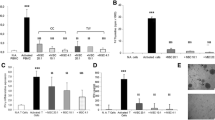
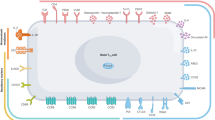
Abstract
This chapter describes ex vivo isolation of human T cells and of naïve splenocytes respectively collected from multiple sclerosis patients and healthy controls and experimental autoimmune encephalomyelitis-affected mice. After the magnetic sorting of naïve and activated T helper lymphocytes, we provide details about the cell cultures to measure the interaction with extracellular matrix proteins using standard cell invasion or hand-made in vitro assays, upon different stimuli, through Toll-like receptor(s) ligands, T-cell activators, and cell adhesion molecules modulators. Finally, we describe the methods to harvest and recover T cells to evaluate the properties associated with their trafficking ability.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Nicolò C, Di Sante G, Orsini M et al (2006) Mycobacterium tuberculosis in the adjuvant modulates the balance of Th immune response to self-antigen of the CNS without influencing a “core” repertoire of specific T cells. Int Immunol 18:363–374. https://doi.org/10.1093/intimm/dxh376
Nicolò C, Sali M, Di Sante G et al (2010) Mycobacterium smegmatis expressing a chimeric protein MPT64-proteolipid protein (PLP) 139-151 reorganizes the PLP-specific T cell repertoire favoring a CD8-mediated response and induces a relapsing experimental autoimmune encephalomyelitis. J Immunol 184:222–235. https://doi.org/10.4049/jimmunol.0804263
Nicolò C, Di Sante G, Procoli A et al (2013) M tuberculosis in the adjuvant modulates time of appearance of CNS-specific effector T cells in the spleen through a polymorphic site of TLR2. PLoS One 8:e55819. https://doi.org/10.1371/journal.pone.0055819
Penitente R, Nicolò C, Van den Elzen P et al (2008) Administration of PLP 139–151 primes T cells distinct from those spontaneously responsive in vitro to this antigen. J Immunol 180:6611–6622. https://doi.org/10.4049/jimmunol.180.10.6611
Piermattei A, Migliara G, Di Sante G et al (2016) Toll-like receptor 2 mediates in vivo pro- and anti-inflammatory effects of mycobacterium tuberculosis and modulates autoimmune encephalomyelitis. Front Immunol 7:191. https://doi.org/10.3389/fimmu.2016.00191
Tredicine M, Camponeschi C, Pirolli D et al (2022) A TLR/CD44 axis regulates T cell trafficking in experimental and human multiple sclerosis. iScience 25:103763. https://doi.org/10.1016/j.isci.2022.103763
Oukka M, Bettelli E (2018) Regulation of lymphocyte trafficking in central nervous system autoimmunity. Curr Opin Immunol 55:38–43. https://doi.org/10.1016/j.coi.2018.09.008
Sandor AM, Jacobelli J, Friedman RS (2019) Immune cell trafficking to the islets during type 1 diabetes. Clin Exp Immunol 198:314–325. https://doi.org/10.1111/cei.13353
Strazza M, Azoulay-Alfaguter I, Silverman GJ, Mor A (2015) T cell chemokine receptor patterns as pathogenic signatures in autoimmunity. Discov Med 19:117–125
Calvier L, Demuth G, Manouchehri N et al (2020) Reelin depletion protects against autoimmune encephalomyelitis by decreasing vascular adhesion of leukocytes. Sci Transl Med 12:eaay7675. https://doi.org/10.1126/scitranslmed.aay7675
Mousavi A (2020) CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy. Immunol Lett 217:91–115. https://doi.org/10.1016/j.imlet.2019.11.007
Gross CC, Schulte-Mecklenbeck A, Hanning U et al (2017) Distinct pattern of lesion distribution in multiple sclerosis is associated with different circulating T-helper and helper-like innate lymphoid cell subsets. Mult Scler J 23:1025–1030. https://doi.org/10.1177/1352458516662726
Lindner M, Klotz L, Wiendl H (2018) Mechanisms underlying lesion development and lesion distribution in CNS autoimmunity. J Neurochem 146:122–132. https://doi.org/10.1111/jnc.14339
Visser L, Melief M-J, van Riel D et al (2006) Phagocytes containing a disease-promoting toll-like receptor/nod ligand are present in the brain during demyelinating disease in primates. Am J Pathol 169:1671–1685. https://doi.org/10.2353/ajpath.2006.060143
Visser L, Jan de Heer H, Boven LA et al (2005) Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol 174:808–816. https://doi.org/10.4049/jimmunol.174.2.808
Schrijver IA, van Meurs M, Melief MJ et al (2001) Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain 124:1544–1554. https://doi.org/10.1093/brain/124.8.1544
Jagessar SA, Kap YS, Heijmans N et al (2010) Induction of progressive demyelinating autoimmune encephalomyelitis in common marmoset monkeys using MOG 34-56 peptide in incomplete freund adjuvant. J Neuropathol Exp Neurol 69:372–385. https://doi.org/10.1097/NEN.0b013e3181d5d053
Shaw PJ, Barr MJ, Lukens JR et al (2011) Signaling via the RIP2 adaptor protein in central nervous system-infiltrating dendritic cells promotes inflammation and autoimmunity. Immunity 34:75–84. https://doi.org/10.1016/j.immuni.2010.12.015
Beura LK, Hamilton SE, Bi K et al (2016) Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532:512–516. https://doi.org/10.1038/nature17655
Valentini M, Piermattei A, Di Sante G et al (2014) Immunomodulation by gut microbiota: role of toll-like receptor expressed by T cells. J Immunol Res 2014:586939. https://doi.org/10.1155/2014/586939
Kuzmich NN, Sivak KV, Chubarev VN et al (2017) TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel) 5. https://doi.org/10.3390/vaccines5040034
Luz A, Fainstein N, Einstein O, Ben-Hur T (2015) The role of CNS TLR2 activation in mediating innate versus adaptive neuroinflammation. Exp Neurol 273:234–242. https://doi.org/10.1016/j.expneurol.2015.08.021
Ko R, Park JH, Ha H et al (2015) Glycogen synthase kinase 3β ubiquitination by TRAF6 regulates TLR3-mediated pro-inflammatory cytokine production. Nat Commun 6:6765. https://doi.org/10.1038/ncomms7765
Stierschneider A, Neuditschko B, Colleselli K, Hundsberger H, Herzog F, Wiesner C (2023) Comparative and temporal characterization of LPS and Blue-Light-Induced TLR4 signal transduction and gene expression in optogenetically manipulated endothelial cells. Cells 12:697
Fallarino F, Gargaro M, Mondanell G et al (2016) Delineating the role of toll-like receptors in the neuro-inflammation model EAE. Methods Mol Biol 1390:383–411. https://doi.org/10.1007/978-1-4939-3335-8_23
Colleselli K, Ebeyer-Masotta M, Neuditschko B, Stierschneider A, Pollhammer C, Potocnjak M, Hundsberger H, Herzog F, Wiesner C (2023) Beyond pattern recognition: TLR2 promotes chemotaxis, cell adhesion and migration in THP-1 cells. Cells 12:1425. https://doi.org/10.3390/cells12101425
Brennan FR, Mikecz K, Glant TT et al (1997) CD44 expression by leucocytes in rheumatoid arthritis and modulation by specific antibody: implications for lymphocyte adhesion to endothelial cells and synoviocytes in vitro. Scand J Immunol 45:213–220. https://doi.org/10.1046/j.1365-3083.1997.d01-382.x
McDonald B, Kubes P (2015) Interactions between CD44 and Hyaluronan in leukocyte trafficking. Front Immunol 6:68. https://doi.org/10.3389/fimmu.2015.00068
Ghazi-Visser L, Laman JD, Nagel S et al (2013) CD44 variant isoforms control experimental autoimmune encephalomyelitis by affecting the lifespan of the pathogenic T cells. FASEB J 27:3683–3701. https://doi.org/10.1096/fj.13-228809
Laman JD, Maassen CB, Schellekens MM et al (1998) Therapy with antibodies against CD40L (CD154) and CD44-variant isoforms reduces experimental autoimmune encephalomyelitis induced by a proteolipid protein peptide. Mult Scler 4:147–153. https://doi.org/10.1177/135245859800400312
Laman JD, ‘t Hart BA, Power C, Dziarski R (2020) Bacterial peptidoglycan as a driver of chronic brain inflammation. Trends Mol Med 26:670–682. https://doi.org/10.1016/j.molmed.2019.11.006
Yang C, Liang H, Zhao H, Jiang X (2012) CD44 variant isoforms are specifically expressed on peripheral blood lymphocytes from asthmatic patients. Exp Ther Med 4:79–83. https://doi.org/10.3892/etm.2012.543
Latini A, Novelli L, Ceccarelli F et al (2021) mRNA expression analysis confirms CD44 splicing impairment in systemic lupus erythematosus patients. Lupus 30:1086–1093. https://doi.org/10.1177/09612033211004725
Novelli L, Barbati C, Ceccarelli F et al (2019) CD44v3 and CD44v6 isoforms on T cells are able to discriminate different disease activity degrees and phenotypes in systemic lupus erythematosus patients. Lupus 28:621–628. https://doi.org/10.1177/0961203319838063
Camponeschi C, De Carluccio M, Amadio S et al (2021) S100B protein as a therapeutic target in multiple sclerosis: the S100B inhibitor Arundic acid protects from chronic experimental autoimmune encephalomyelitis. IJMS 22:13558. https://doi.org/10.3390/ijms222413558
Miller SD, Karpus WJ, Davidson TS (2010) Experimental autoimmune encephalomyelitis in the mouse. In: Coligan JE, Bierer BE, Margulies DH et al (eds) Current protocols in immunology, vol 88. Wiley, Hoboken
Stromnes IM, Goverman JM (2006) Active induction of experimental allergic encephalomyelitis. Nat Protoc 1:1810–1819. https://doi.org/10.1038/nprot.2006.285
Di Sante G, Gremese E, Tolusso B et al (2021) Haemophilus parasuis (Glaesserella parasuis) as a potential driver of molecular mimicry and inflammation in rheumatoid arthritis. Front Med (Lausanne) 8:671018. https://doi.org/10.3389/fmed.2021.671018
Di Sante G, Tolusso B, Fedele AL et al (2015) Collagen specific T-cell repertoire and HLA-DR alleles: biomarkers of active refractory rheumatoid arthritis. EBioMedicine 2:2037–2045. https://doi.org/10.1016/j.ebiom.2015.11.019
Marino M, Maiuri MT, Di Sante G et al (2014) T cell repertoire in DQ5-positive MuSK-positive myasthenia gravis patients. J Autoimmun 52:113–121. https://doi.org/10.1016/j.jaut.2013.12.007
Di Sante G, Amadio S, Sampaolese B et al (2020) The S100B inhibitor pentamidine ameliorates clinical score and neuropathology of relapsing—remitting multiple sclerosis mouse model. Cell 9:748. https://doi.org/10.3390/cells9030748
Marchese E, Valentini M, Sante GD et al (2020) Alternative splicing of neurexins 1-3 is modulated by neuroinflammation in the prefrontal cortex of a murine model of multiple sclerosis. Exp Neurol:113497. https://doi.org/10.1016/j.expneurol.2020.113497
Simmons SB, Pierson ER, Lee SY, Goverman JM (2013) Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol 34:410–422. https://doi.org/10.1016/j.it.2013.04.006
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1:87–93. https://doi.org/10.4103/0976-500X.72350
Stone NL, England TJ, O’Sullivan SE (2019) A novel transwell blood brain barrier model using primary human cells. Front Cell Neurosci 13:230. https://doi.org/10.3389/fncel.2019.00230
Thomsen MS, Humle N, Hede E et al (2021) The blood-brain barrier studied in vitro across species. PLoS One 16:e0236770. https://doi.org/10.1371/journal.pone.0236770
Schroeter CB, Herrmann AM, Bock S et al (2021) One brain—all cells: a comprehensive protocol to isolate all principal CNS-resident cell types from brain and spinal cord of adult healthy and EAE mice. Cell 10:651. https://doi.org/10.3390/cells10030651
Acknowledgments
This work received nonconditional support by Fondazione Cassa di Risparmio di Perugia, Project 2021.0347 (GDS).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Moliterni, C. et al. (2023). In Vitro and Ex Vivo Methodologies for T-Cell Trafficking Through Blood–Brain Barrier After TLR Activation. In: Fallarino, F., Gargaro, M., Manni, G. (eds) Toll-Like Receptors. Methods in Molecular Biology, vol 2700. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3366-3_12
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3366-3_12
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3365-6
Online ISBN: 978-1-0716-3366-3
eBook Packages: Springer Protocols