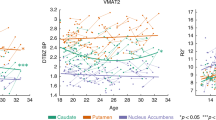
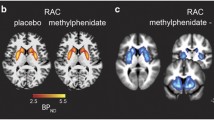
Abstract
Measures of dopamine (DA) in vivo in humans have been obtained using positron emission tomography (PET), however, its invasive nature and exposure to radioactivity has limited its application to populations such as pediatric cohorts. Brain tissue iron measured noninvasively with magnetic resonance imaging (MRI) has been found to be linked to DA neurophysiology in the basal ganglia, providing a novel approach for in vivo human characterization of DA function. This chapter describes the links between brain tissue iron and DA neurophysiology, the MRI approaches available to measure it, and application to understanding development and disease. The literature characterizing brain tissue iron in DA neurophysiology and new advances in MRI approaches to measure it support this as an innovative and promising approach to understand the DAergic mechanisms underlying lifespan development and disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Luciana M, Wahlstrom D, Porter JN, Collins PF (2012) Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev Psychol 48(3):844–861. https://doi.org/10.1037/a0027432
Wahlstrom D, White T, Luciana M (2010) Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev 34(5):631–648. https://doi.org/10.1016/j.neubiorev.2009.12.007
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry 69(8):776–786. https://doi.org/10.1001/archgenpsychiatry.2012.169
Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9(12):947–957. https://doi.org/10.1038/nrn2513
Davey CG, Yücel M, Allen NB (2008) The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev 32(1):1–19. https://doi.org/10.1016/j.neubiorev.2007.04.016
Diehl DJ, Gershon S (1992) The role of dopamine in mood disorders. Compr Psychiatry 33(2):115–120. https://doi.org/10.1016/0010-440X(92)90007-D
Dionelis K, Meng C, Craig K, Shabbir S, Fineberg N, Sahakian B, Suckling J, Bullmore E, Robbins T, Ersche K (2019) Dopaminergic modulation of frontostriatal networks in disorders of addiction and compulsion. Eur Neuropsychopharmacol 29:S490–S491. https://doi.org/10.17863/CAM.48474
Ernst M, Luciana M (2015) Neuroimaging of the dopamine/reward system in adolescent drug use. CNS Spectr 20(4):427–441. https://doi.org/10.1017/S1092852915000395
Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, Banich MT (2018) Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci 32:67–79. https://doi.org/10.1016/j.dcn.2018.02.006
Tervo-Clemmens B, Quach A, Calabro FJ, Foran W, Luna B (2020) Meta-analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use. NeuroImage 209:116476. https://doi.org/10.1016/j.neuroimage.2019.116476
Alakurtti K, Johansson JJ, Joutsa J, Laine M, Bäckman L, Nyberg L, Rinne JO (2015) Long-term test–retest reliability of striatal and extrastriatal dopamine D2/3 receptor binding: study with [11C]raclopride and high-resolution PET. J Cereb Blood Flow Metab 35(7):1199–1205. https://doi.org/10.1038/jcbfm.2015.53
Farde L, Hall H, Pauli S, Halldin C (1995) Variability in D2-dopamine receptor density and affinity: a PET study with [11C]raclopride in man. Synapse 20(3):200–208. https://doi.org/10.1002/syn.890200303
Kilbourn MR (2014) Radioligands for imaging vesicular monoamine transporters. In: Dierckx RAJO, Otte A, de Vries EFJ, van Waarde A, Luiten PGM (eds) PET and SPECT of neurobiological systems. Springer, pp 765–790. https://doi.org/10.1007/978-3-642-42014-6_27
Knutson B, Gibbs SEB (2007) Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology 191(3):813–822. https://doi.org/10.1007/s00213-006-0686-7
Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW (2004) Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci 24(8):1793–1802. https://doi.org/10.1523/JNEUROSCI.4862-03.2004
Bjork JM, Smith AR, Chen G, Hommer DW (2010) Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One 5(7):e11440. https://doi.org/10.1371/journal.pone.0011440
Braams BR, van Duijvenvoorde ACK, Peper JS, Crone EA (2015) Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J Neurosci 35(18):7226–7238. https://doi.org/10.1523/JNEUROSCI.4764-14.2015
Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS (2005) Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage 25(4):1279–1291. https://doi.org/10.1016/j.neuroimage.2004.12.038
Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ (2006) Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci 26(25):6885–6892. https://doi.org/10.1523/JNEUROSCI.1062-06.2006
Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B (2010) Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex 20(7):1613–1629. https://doi.org/10.1093/cercor/bhp225
Luna B, Paulsen DJ, Padmanabhan A, Geier C (2013) Cognitive control and motivation. Curr Dir Psychol Sci 22(2):94–100. https://doi.org/10.1177/0963721413478416
Padmanabhan A (2011) Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci 1:517–529
Paulsen DJ, Hallquist MN, Geier CF, Luna B (2015) Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Dev Cogn Neurosci 11:105–115. https://doi.org/10.1016/j.dcn.2014.09.003
Hillman EMC (2014) Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 37(1):161–181. https://doi.org/10.1146/annurev-neuro-071013-014111
Logothetis NK, Wandell BA (2004) Interpreting the BOLD signal. Annu Rev Physiol 66(1):735–769. https://doi.org/10.1146/annurev.physiol.66.082602.092845
Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, Flor H, Braus DF, Buchholz HG, Gründer G, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P (2004) Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatr 161(10):1783–1789. https://doi.org/10.1176/ajp.161.10.1783
Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Röcken M, Nutt RE, Machulla H-J, Uludag K, Cherry SR, Claussen CD, Pichler BJ (2008) Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med 14(4):459–465. https://doi.org/10.1038/nm1700
Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze H-J, Zilles K, Düzel E, Bauer A (2008) Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci 28(52):14311–14319. https://doi.org/10.1523/JNEUROSCI.2058-08.2008
Brocka M, Helbing C, Vincenz D, Scherf T, Montag D, Goldschmidt J, Angenstein F, Lippert M (2018) Contributions of dopaminergic and non-dopaminergic neurons to VTA-stimulation induced neurovascular responses in brain reward circuits. NeuroImage 177:88–97. https://doi.org/10.1016/j.neuroimage.2018.04.059
Attwell D, Iadecola C (2002) The neural basis of functional brain imaging signals. Trends Neurosci 25(12):621–625. https://doi.org/10.1016/S0166-2236(02)02264-6
Logothetis NK (2003) The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 23(10):3963–3971. https://doi.org/10.1523/JNEUROSCI.23-10-03963.2003
Jongkees BJ, Colzato LS (2016) Spontaneous eye blink rate as predictor of dopamine-related cognitive function—a review. Neurosci Biobehav Rev 71:58–82. https://doi.org/10.1016/j.neubiorev.2016.08.020
Larsen B, Olafsson V, Calabro F, Laymon C, Tervo-Clemmens B, Campbell E, Minhas D, Montez D, Price J, Luna B (2020) Maturation of the human striatal dopamine system revealed by PET and quantitative MRI. Nat Commun 11(1):846. https://doi.org/10.1038/s41467-020-14693-3
Connor JR, Menzies SL (1996) Relationship of iron to oligodendrocytes and myelination. Glia 17(2):83–93. https://doi.org/10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13(10):1045–1060. https://doi.org/10.1016/S1474-4422(14)70117-6
Rouault TA (2013) Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 14(8):551–564. https://doi.org/10.1038/nrn3453
Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR (2009) Oligodendrocytes and myelination: the role of iron. Glia 57(5):467–478. https://doi.org/10.1002/glia.20784
Ramsey AJ, Hillas PJ, Fitzpatrick PF (1996) Characterization of the active site iron in tyrosine hydroxylase. J Biol Chem 271(40):24395–24400. https://doi.org/10.1074/jbc.271.40.24395
Lu H, Chen J, Huang H, Zhou M, Zhu Q, Yao SQ, Chai Z, Hu Y (2017) Iron modulates the activity of monoamine oxidase B in SH-SY5Y cells. Biometals 30(4):599–607. https://doi.org/10.1007/s10534-017-0030-1
Youdim MBH (2018) Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases. J Neural Transm 125(11):1719–1733. https://doi.org/10.1007/s00702-018-1942-9
Youdim MBH, Grahame-Smith DG, Woods HF (1976) Some properties of human platelet monoamine oxidase in iron-deficiency anaemia. Clin Sci Mol Med 50(6):479–485. https://doi.org/10.1042/cs0500479
Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 155:96–119. https://doi.org/10.1016/j.pneurobio.2015.09.012
Ortega R, Cloetens P, Devès G, Carmona A, Bohic S (2007) Iron storage within dopamine neurovesicles revealed by chemical nano-imaging. PLoS One 2(9):e925. https://doi.org/10.1371/journal.pone.0000925
Brass SD, Chen N, Mulkern RV, Bakshi R (2006) Magnetic resonance imaging of iron deposition in neurological disorders. Top Magn Reson Imaging 17(1):31–40. https://doi.org/10.1097/01.rmr.0000245459.82782.e4
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3(1):41–51. https://doi.org/10.1111/j.1471-4159.1958.tb12607.x
Thomas LO, Boyko OB, Anthony DC, Burger PC (1993) MR detection of brain iron. Am J Neuroradiol 14(5):1043–1048
Adisetiyo V, Jensen JH, Tabesh A, Deardorff RL, Fieremans E, Di Martino A, Gray KM, Castellanos FX, Helpern JA (2014) Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment? Radiology 272(2):524–532. https://doi.org/10.1148/radiol.14140047
Allen RP, Earley CJ (2007) The role of iron in restless legs syndrome. Mov Disord 22(Suppl 18):S440–S448. https://doi.org/10.1002/mds.21607
Bartzokis G, Tishler TA, Shin I-S, Lu PH, Cummings JL (2004) Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann N Y Acad Sci 1012(1):224–236. https://doi.org/10.1196/annals.1306.019
Khan FH, Ahlberg CD, Chow CA, Shah DR, Koo BB (2017) Iron, dopamine, genetics, and hormones in the pathophysiology of restless legs syndrome. J Neurol 264(8):1634–1641. https://doi.org/10.1007/s00415-017-8431-1
Piao Y-S, Lian T-H, Hu Y, Zuo L-J, Guo P, Yu S-Y, Liu L, Jin Z, Zhao H, Li L-X, Yu Q-J, Wang R-D, Chen S-D, Chan P, Wang X-M, Zhang W (2017) Restless legs syndrome in Parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci Rep 7:10547. https://doi.org/10.1038/s41598-017-10593-7
Connor JR, Wang X-S, Allen RP, Beard JL, Wiesinger JA, Felt BT, Earley CJ (2009) Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 132(9):2403–2412. https://doi.org/10.1093/brain/awp125
Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas R, Brasic J, Ravert HT, Dannals RF, Allen RP (2011) The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep 34(3):341–347. https://doi.org/10.1093/sleep/34.3.341
Earley CJ, Connor J, Garcia-Borreguero D, Jenner P, Winkelman J, Zee PC, Allen R (2014) Altered brain iron homeostasis and dopaminergic function in restless legs syndrome (Willis–Ekbom disease). Sleep Med 15(11):1288–1301. https://doi.org/10.1016/j.sleep.2014.05.009
Unger EL, Bianco LE, Jones BC, Allen RP, Earley CJ (2014) Low brain iron effects and reversibility on striatal dopamine dynamics. Exp Neurol 261:462–468. https://doi.org/10.1016/j.expneurol.2014.06.023
Ersche KD, Acosta-Cabronero J, Jones PS, Ziauddeen H, van Swelm RPL, Laarakkers CMM, Raha-Chowdhury R, Williams GB (2017) Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl Psychiatry 7(2):e1040. https://doi.org/10.1038/tp.2016.271
Beard J (2003) Iron deficiency alters brain development and functioning. J Nutr 133(5):1468S–1472S. https://doi.org/10.1093/jn/133.5.1468S
Beard JL, Erikson KM, Jones BC (2002) Neurobehavioral analysis of developmental iron deficiency in rats. Behav Brain Res 134(1):517–524. https://doi.org/10.1016/S0166-4328(02)00092-X
Erikson KM, Jones BC, Beard JL (2000) Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr 130(11):2831–2837. https://doi.org/10.1093/jn/130.11.2831
Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL (2001) Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav 69(3):409–418. https://doi.org/10.1016/S0091-3057(01)00563-9
Jellen LC, Lu L, Wang X, Unger EL, Earley CJ, Allen RP, Williams RW, Jones BC (2013) Iron deficiency alters expression of dopamine-related genes in the ventral midbrain in mice. Neuroscience 252:13–23. https://doi.org/10.1016/j.neuroscience.2013.07.058
Lozoff B (2011) Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr 141(4):740S–746S. https://doi.org/10.3945/jn.110.131169
Unger EL, Wiesinger JA, Hao L, Beard JL (2008) Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J Nutr 138(12):2487–2494. https://doi.org/10.3945/jn.108.095224
Wiesinger JA, Buwen JP, Cifelli CJ, Unger EL, Jones BC, Beard JL (2007) Down-regulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis. J Neurochem 100(1):167–179. https://doi.org/10.1111/j.1471-4159.2006.04175.x
Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L (2009) Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology 252(1):165–172. https://doi.org/10.1148/radiol.2522081399
Hect JL, Daugherty AM, Hermez KM, Thomason ME (2018) Developmental variation in regional brain iron and its relation to cognitive functions in childhood. Dev Cogn Neurosci 34:18–26. https://doi.org/10.1016/j.dcn.2018.05.004
Larsen B, Bourque J, Moore TM, Adebimpe A, Calkins ME, Elliott MA, Gur RC, Gur RE, Moberg PJ, Roalf DR, Ruparel K, Turetsky BI, Vandekar SN, Wolf DH, Shinohara RT, Satterthwaite TD (2020) Longitudinal development of brain iron is linked to cognition in youth. J Neurosci 40(9):1810–1818. https://doi.org/10.1523/JNEUROSCI.2434-19.2020
Larsen B, Luna B (2015) In vivo evidence of neurophysiological maturation of the human adolescent striatum. Dev Cogn Neurosci 12:74–85. https://doi.org/10.1016/j.dcn.2014.12.003
Peterson ET, Kwon D, Luna B, Larsen B, Prouty D, Bellis MDD, Voyvodic J, Liu C, Li W, Pohl KM, Sullivan EV, Pfefferbaum A (2019) Distribution of brain iron accrual in adolescence: evidence from cross-sectional and longitudinal analysis. Hum Brain Mapp 40(5):1480–1495. https://doi.org/10.1002/hbm.24461
Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA (1986) MRI of brain iron. AJR Am J Roentgenol 147(1):103–110. https://doi.org/10.2214/ajr.147.1.103
Hametner S, Endmayr V, Deistung A, Palmrich P, Prihoda M, Haimburger E, Menard C, Feng X, Haider T, Leisser M, Köck U, Kaider A, Höftberger R, Robinson S, Reichenbach JR, Lassmann H, Traxler H, Trattnig S, Grabner G (2018) The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation—a biochemical and histological validation study. NeuroImage 179:117–133. https://doi.org/10.1016/j.neuroimage.2018.06.007
Langkammer C, Krebs N, Goessler W, Scheurer E, Ebner F, Yen K, Fazekas F, Ropele S (2010) Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257(2):455–462. https://doi.org/10.1148/radiol.10100495
Stüber C, Morawski M, Schäfer A, Labadie C, Wähnert M, Leuze C, Streicher M, Barapatre N, Reimann K, Geyer S, Spemann D, Turner R (2014) Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. NeuroImage 93:95–106. https://doi.org/10.1016/j.neuroimage.2014.02.026
Haacke EM, Cheng NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23(1):1–25. https://doi.org/10.1016/j.mri.2004.10.001
Haacke EM, Miao Y, Liu M, Habib CA, Katkuri Y, Liu T, Yang Z, Lang Z, Hu J, Wu J (2010) Correlation of putative iron content as represented by changes in R2* and phase with age in deep gray matter of healthy adults. J Magn Reson Imaging 32(3):561–576. https://doi.org/10.1002/jmri.22293
Ma J, Wehrli FW (1996) Method for image-based measurement of the reversible and irreversible contribution to the transverse-relaxation rate. J Magn Reson Ser B 111(1):61–69. https://doi.org/10.1006/jmrb.1996.0060
Wang Y, Liu T (2015) Quantitative susceptibility mapping (QSM): decoding MRI data for a tissue magnetic biomarker. Magn Reson Med 73(1):82–101. https://doi.org/10.1002/mrm.25358
Sedlacik J, Boelmans K, Löbel U, Holst B, Siemonsen S, Fiehler J (2014) Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T. NeuroImage 84:1032–1041. https://doi.org/10.1016/j.neuroimage.2013.08.051
Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM (2009) Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 29(5):1433–1449. https://doi.org/10.1148/rg.295095034
Yablonskiy DA, Haacke EM (1994) Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 32(6):749–763. https://doi.org/10.1002/mrm.1910320610
Deistung A, Schäfer A, Schweser F, Biedermann U, Güllmar D, Trampel R, Turner R, Reichenbach JR (2013) High-resolution MR imaging of the human brainstem in vivo at 7 Tesla. Front Hum Neurosci 7:710. https://doi.org/10.3389/fnhum.2013.00710
Péran P, Hagberg G, Luccichenti G, Cherubini A, Brainovich V, Celsis P, Caltagirone C, Sabatini U (2007) Voxel-based analysis of R2* maps in the healthy human brain. J Magn Reson Imaging 26(6):1413–1420. https://doi.org/10.1002/jmri.21204
Esterhammer R, Seppi K, Reiter E, Pinter B, Mueller C, Kremser C, Zitzelsberger T, Nocker M, Scherfler C, Poewe W, Schocke M (2015) Potential of diffusion tensor imaging and relaxometry for the detection of specific pathological alterations in Parkinson’s disease (PD). PLoS One 10(12):e0145493. https://doi.org/10.1371/journal.pone.0145493
Friedrich I, Reimann K, Jankuhn S, Kirilina E, Stieler J, Sonntag M, Meijer J, Weiskopf N, Reinert T, Arendt T, Morawski M (2021) Cell specific quantitative iron mapping on brain slices by immuno-μPIXE in healthy elderly and Parkinson’s disease. Acta Neuropathol Commun 9(1):47. https://doi.org/10.1186/s40478-021-01145-2
Gorell JM, Ordidge RJ, Brown GG, Deniau J-C, Buderer NM, Helpern JA (1995) Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology 45(6):1138–1143. https://doi.org/10.1212/WNL.45.6.1138
Hopes L, Grolez G, Moreau C, Lopes R, Ryckewaert G, Carrière N, Auger F, Laloux C, Petrault M, Devedjian J-C, Bordet R, Defebvre L, Jissendi P, Delmaire C, Devos D (2016) Magnetic resonance imaging features of the nigrostriatal system: biomarkers of Parkinson’s disease stages? PLoS One 11(4):e0147947. https://doi.org/10.1371/journal.pone.0147947
Rossi M, Ruottinen H, Soimakallio S, Elovaara I, Dastidar P (2013) Clinical MRI for iron detection in Parkinson’s disease. Clin Imaging 37(4):631–636. https://doi.org/10.1016/j.clinimag.2013.02.001
Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F (2013) Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PLoS One 8(3):e57904. https://doi.org/10.1371/journal.pone.0057904
Wieler M, Gee M, Martin WRW (2015) Longitudinal midbrain changes in early Parkinson’s disease: iron content estimated from R2*/MRI. Parkinsonism Relat Disord 21(3):179–183. https://doi.org/10.1016/j.parkreldis.2014.11.017
Zhang Y, Gauthier SA, Gupta A, Chen W, Comunale J, Chiang GC-Y, Zhou D, Askin G, Zhu W, Pitt D, Wang Y (2016) Quantitative susceptibility mapping and R2* measured changes during white matter lesion development in multiple sclerosis: myelin breakdown, myelin debris degradation and removal, and iron accumulation. Am J Neuroradiol 37(9):1629–1635. https://doi.org/10.3174/ajnr.A4825
Betts MJ, Acosta-Cabronero J, Cardenas-Blanco A, Nestor PJ, Düzel E (2016) High-resolution characterisation of the aging brain using simultaneous quantitative susceptibility mapping (QSM) and R2* measurements at 7T. NeuroImage 138:43–63. https://doi.org/10.1016/j.neuroimage.2016.05.024
Brammerloh M, Morawski M, Friedrich I, Reinert T, Lange C, Pelicon P, Vavpetič P, Jankuhn S, Jäger C, Alkemade A, Balesar R, Pine K, Gavriilidis F, Trampel R, Reimer E, Arendt T, Weiskopf N, Kirilina E (2021) Measuring the iron content of dopaminergic neurons in substantia nigra with MRI relaxometry. NeuroImage 239:118255. https://doi.org/10.1016/j.neuroimage.2021.118255
Gelman N, Gorell JM, Barker PB, Savage RM, Spickler EM, Windham JP, Knight RA (1999) MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 210(3):759–767. https://doi.org/10.1148/radiology.210.3.r99fe41759
Wehrli FW, MacFall JR, Glover GH, Grigsby N, Haughton V, Johanson J (1984) The dependence of nuclear magnetic resonance (NMR) image contrast on intrinsic and pulse sequence timing parameters. Magn Reson Imaging 2(1):3–16. https://doi.org/10.1016/0730-725X(84)90119-X
Yablonskiy DA (1998) Quantitation of intrinsic magnetic susceptibility-related effects in a tissue matrix. Phantom study. Magn Reson Med 39(3):417–428. https://doi.org/10.1002/mrm.1910390312
Graham JM, Paley MNJ, Grünewald RA, Hoggard N, Griffiths PD (2000) Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain 123(12):2423–2431. https://doi.org/10.1093/brain/123.12.2423
Miszkiel KA, Paley MNJ, Wilkinson ID, Hall-Craggs MA, Ordidge R, Kendall BE, Miller RF, Harrison MJG (1997) The measurement of R2, R2* and R2′ in HIV-infected patients using the prime sequence as a measure of brain iron deposition. Magn Reson Imaging 15(10):1113–1119. https://doi.org/10.1016/S0730-725X(97)00089-1
Ghassaban K, Liu S, Jiang C, Haacke EM (2019) Quantifying iron content in magnetic resonance imaging. NeuroImage 187:77–92. https://doi.org/10.1016/j.neuroimage.2018.04.047
Möller HE, Bossoni L, Connor JR, Crichton RR, Does MD, Ward RJ, Zecca L, Zucca FA, Ronen I (2019) Iron, myelin, and the brain: neuroimaging meets neurobiology. Trends Neurosci 42(6):384–401. https://doi.org/10.1016/j.tins.2019.03.009
Daugherty AM, Hoagey DA, Kennedy KM, Rodrigue KM (2019) Genetic predisposition for inflammation exacerbates effects of striatal iron content on cognitive switching ability in healthy aging. NeuroImage 185:471–478. https://doi.org/10.1016/j.neuroimage.2018.10.064
Salami A, Avelar-Pereira B, Garzón B, Sitnikov R, Kalpouzos G (2018) Functional coherence of striatal resting-state networks is modulated by striatal iron content. NeuroImage 183:495–503. https://doi.org/10.1016/j.neuroimage.2018.08.036
Parr AC, Calabro F, Larsen B, Tervo-Clemmens B, Elliot S, Foran W, Olafsson V, Luna B (2021) Dopamine-related striatal neurophysiology is associated with specialization of frontostriatal reward circuitry through adolescence. Prog Neurobiol 201:101997. https://doi.org/10.1016/j.pneurobio.2021.101997
Deistung A, Rauscher A, Sedlacik J, Stadler J, Witoszynskyj S, Reichenbach JR (2008) Susceptibility weighted imaging at ultra high magnetic field strengths: theoretical considerations and experimental results. Magn Reson Med 60(5):1155–1168. https://doi.org/10.1002/mrm.21754
Bender B, Klose U (2010) The in vivo influence of white matter fiber orientation towards B0 on T2* in the human brain. NMR Biomed 23(9):1071–1076. https://doi.org/10.1002/nbm.1534
Daugherty A, Raz N (2013) Age-related differences in iron content of subcortical nuclei observed in vivo: a meta-analysis. NeuroImage 70:113–121. https://doi.org/10.1016/j.neuroimage.2012.12.040
Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, Sommer K, Reishofer G, Yen K, Fazekas F, Ropele S, Reichenbach JR (2012) Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage 62(3):1593–1599. https://doi.org/10.1016/j.neuroimage.2012.05.049
Schenck JF (2003) Magnetic resonance imaging of brain iron. J Neurol Sci 207(1):99–102. https://doi.org/10.1016/S0022-510X(02)00431-8
Aoki S, Okada Y, Nishimura K, Barkovich AJ, Kjos BO, Brasch RC, Norman D (1989) Normal deposition of brain iron in childhood and adolescence: MR imaging at 1.5 T. Radiology 172(2):381–385. https://doi.org/10.1148/radiology.172.2.2748819
Gossuin Y, Muller RN, Gillis P (2004) Relaxation induced by ferritin: a better understanding for an improved MRI iron quantification. NMR Biomed 17(7):427–432. https://doi.org/10.1002/nbm.903
Denk C, Torres EH, MacKay A, Rauscher A (2011) The influence of white matter fibre orientation on MR signal phase and decay. NMR Biomed 24(3):246–252. https://doi.org/10.1002/nbm.1581
He X, Yablonskiy DA (2009) Biophysical mechanisms of phase contrast in gradient echo MRI. Proc Natl Acad Sci 106(32):13558–13563. https://doi.org/10.1073/pnas.0904899106
Lee J, van Gelderen P, Kuo L-W, Merkle H, Silva AC, Duyn JH (2011) T2*-based fiber orientation mapping. NeuroImage 57(1):225–234. https://doi.org/10.1016/j.neuroimage.2011.04.026
Li W, Wu B, Avram AV, Liu C (2012) Magnetic susceptibility anisotropy of human brain in vivo and its molecular underpinnings. NeuroImage 59(3):2088–2097. https://doi.org/10.1016/j.neuroimage.2011.10.038
Rudko DA, Solovey I, Gati JS, Kremenchutzky M, Menon RS (2014) Multiple sclerosis: improved identification of disease-relevant changes in gray and white matter by using susceptibility-based MR imaging. Radiology 272(3):851–864. https://doi.org/10.1148/radiol.14132475
Wharton S, Bowtell R (2013) Gradient echo based fiber orientation mapping using R2* and frequency difference measurements. NeuroImage 83:1011–1023. https://doi.org/10.1016/j.neuroimage.2013.07.054
Mitsumori F, Watanabe H, Takaya N (2009) Estimation of brain iron concentration in vivo using a linear relationship between regional iron and apparent transverse relaxation rate of the tissue water at 4.7T. Magn Reson Med 62(5):1326–1330. https://doi.org/10.1002/mrm.22097
Mitsumori F, Watanabe H, Takaya N, Garwood M, Auerbach EJ, Michaeli S, Mangia S (2012) Toward understanding transverse relaxation in human brain through its field dependence. Magn Reson Med 68(3):947–953. https://doi.org/10.1002/mrm.23301
Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22(23):2171–2179. https://doi.org/10.1053/euhj.2001.2822
Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, Deugnier Y (2004) Non-invasive assessment of hepatic iron stores by MRI. Lancet 363(9406):357–362. https://doi.org/10.1016/S0140-6736(04)15436-6
Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y (2015) Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging 33(1):1–25. https://doi.org/10.1016/j.mri.2014.09.004
Yao B, Li T-Q, van Gelderen P, Shmueli K, de Zwart JA, Duyn JH (2009) Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. NeuroImage 44(4):1259–1266. https://doi.org/10.1016/j.neuroimage.2008.10.029
Haacke EM, Xu Y, Cheng Y-CN, Reichenbach JR (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52(3):612–618. https://doi.org/10.1002/mrm.20198
Halefoglu AM, Yousem DM (2018) Susceptibility weighted imaging: clinical applications and future directions. World J Radiol 10(4):30–45. https://doi.org/10.4329/wjr.v10.i4.30
Reichenbach JR, Schweser F, Serres B, Deistung A (2015) Quantitative susceptibility mapping: concepts and applications. Clin Neuroradiol 25(2):225–230. https://doi.org/10.1007/s00062-015-0432-9
Iyer SK, Moon BF, Josselyn N, Ruparel K, Roalf D, Song JW, Guiry S, Ware JB, Kurtz RM, Chawla S, Nabavizadeh SA, Witschey WR (2020) Data-driven quantitative susceptibility mapping using loss adaptive dipole inversion (LADI). J Magn Reson Imaging 52(3):823–835. https://doi.org/10.1002/jmri.27103
Li W, Wu B, Liu C (2011) Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. NeuroImage 55(4):1645–1656. https://doi.org/10.1016/j.neuroimage.2010.11.088
Hopp K, Popescu BFG, McCrea RPE, Harder SL, Robinson CA, Haacke ME, Rajput AH, Rajput A, Nichol H (2010) Brain iron detected by SWI high pass filtered phase calibrated with synchrotron X-ray fluorescence. J Magn Reson Imaging 31(6):1346–1354. https://doi.org/10.1002/jmri.22201
Liu M, Liu S, Ghassaban K, Zheng W, Dicicco D, Miao Y, Habib C, Jazmati T, Haacke EM (2016) Assessing global and regional iron content in deep gray matter as a function of age using susceptibility mapping. J Magn Reson Imaging 44(1):59–71. https://doi.org/10.1002/jmri.25130
Zheng W, Nichol H, Liu S, Cheng Y-CN, Haacke EM (2013) Measuring iron in the brain using quantitative susceptibility mapping and X-ray fluorescence imaging. NeuroImage 78:68–74. https://doi.org/10.1016/j.neuroimage.2013.04.022
Barbosa JHO, Santos AC, Tumas V, Liu M, Zheng W, Haacke EM, Salmon CEG (2015) Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2*. Magn Reson Imaging 33(5):559–565. https://doi.org/10.1016/j.mri.2015.02.021
Li W, Avram AV, Wu B, Xiao X, Liu C (2014) Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed 27(2):219–227. https://doi.org/10.1002/nbm.3056
Li X, Chen L, Kutten K, Ceritoglu C, Li Y, Kang N, Hsu JT, Qiao Y, Wei H, Liu C, Miller MI, Mori S, Yousem DM, van Zijl PCM, Faria AV (2019) Multi-atlas tool for automated segmentation of brain gray matter nuclei and quantification of their magnetic susceptibility. NeuroImage 191:337–349. https://doi.org/10.1016/j.neuroimage.2019.02.016
Schweser F, Deistung A, Lehr BW, Reichenbach JR (2011) Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? NeuroImage 54(4):2789–2807. https://doi.org/10.1016/j.neuroimage.2010.10.070
Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A, Yang S, Nestor PJ (2016) In vivo MRI mapping of brain iron deposition across the adult lifespan. J Neurosci 36(2):364–374. https://doi.org/10.1523/JNEUROSCI.1907-15.2016
Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan EV, Adalsteinsson E (2012) MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. NeuroImage 59(3):2625–2635. https://doi.org/10.1016/j.neuroimage.2011.08.077
Dashtipour K, Liu M, Kani C, Dalaie P, Obenaus A, Simmons D, Gatto NM, Zarifi M (2015) Iron accumulation is not homogenous among patients with Parkinson’s disease. Parkinsons Dis 2015:e324843. https://doi.org/10.1155/2015/324843
Du G, Liu T, Lewis MM, Kong L, Wang Y, Connor J, Mailman RB, Huang X (2016) Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Mov Disord 31(3):317–324. https://doi.org/10.1002/mds.26417
Guan X, Xuan M, Gu Q, Huang P, Liu C, Wang N, Xu X, Luo W, Zhang M (2017) Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed 30(4):e3489. https://doi.org/10.1002/nbm.3489
Guan X, Xu X, Zhang M (2017) Region-specific iron measured by MRI as a biomarker for Parkinson’s disease. Neurosci Bull 33(5):561–567. https://doi.org/10.1007/s12264-017-0138-x
He N, Huang P, Ling H, Langley J, Liu C, Ding B, Huang J, Xu H, Zhang Y, Zhang Z, Hu X, Chen S, Yan F (2017) Dentate nucleus iron deposition is a potential biomarker for tremor-dominant Parkinson’s disease. NMR Biomed 30(4):e3554. https://doi.org/10.1002/nbm.3554
Lotfipour AK, Wharton S, Schwarz ST, Gontu V, Schäfer A, Peters AM, Bowtell RW, Auer DP, Gowland PA, Bajaj NPS (2012) High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson’s disease. J Magn Reson Imaging 35(1):48–55. https://doi.org/10.1002/jmri.22752
Murakami Y, Kakeda S, Watanabe K, Ueda I, Ogasawara A, Moriya J, Ide S, Futatsuya K, Sato T, Okada K, Uozumi T, Tsuji S, Liu T, Wang Y, Korogi Y (2015) Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. Am J Neuroradiol 36(6):1102–1108. https://doi.org/10.3174/ajnr.A4260
Wang Y, Butros SR, Shuai X, Dai Y, Chen C, Liu M, Haacke EM, Hu J, Xu H (2012) Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. Am J Neuroradiol 33(2):266–273. https://doi.org/10.3174/ajnr.A2765
Bian W, Harter K, Hammond-Rosenbluth KE, Lupo JM, Xu D, Kelley DA, Vigneron DB, Nelson SJ, Pelletier D (2013) A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler J 19(1):69–75. https://doi.org/10.1177/1352458512447870
Eissa A, Lebel RM, Korzan JR, Zavodni AE, Warren KG, Catz I, Emery DJ, Wilman AH (2009) Detecting lesions in multiple sclerosis at 4.7 Tesla using phase susceptibility-weighting and T2-weighting. J Magn Reson Imaging 30(4):737–742. https://doi.org/10.1002/jmri.21926
Haacke EM, Makki M, Ge Y, Maheshwari M, Sehgal V, Hu J, Selvan M, Wu Z, Latif Z, Xuan Y, Khan O, Garbern J, Grossman RI (2009) Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J Magn Reson Imaging 29(3):537–544. https://doi.org/10.1002/jmri.21676
Hammond KE, Metcalf M, Carvajal L, Okuda DT, Srinivasan R, Vigneron D, Nelson SJ, Pelletier D (2008) Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol 64(6):707–713. https://doi.org/10.1002/ana.21582
Rumzan R, Wang J, Zeng C, Chen X, Li Y, Luo T, Lv F, Wang Z, Hou H, Huang F (2013) Iron deposition in the precentral grey matter in patients with multiple sclerosis: a quantitative study using susceptibility-weighted imaging. Eur J Radiol 82(2):e95–e99. https://doi.org/10.1016/j.ejrad.2012.09.006
Liu S, Mok K, Neelavalli J, Cheng Y-CN, Tang J, Ye Y, Haacke EM (2014) Improved MR venography using quantitative susceptibility-weighted imaging. J Magn Reson Imaging 40(3):698–708. https://doi.org/10.1002/jmri.24413
Liu C, Li W, Tong KA, Yeom KW, Kuzminski S (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42(1):23–41. https://doi.org/10.1002/jmri.24768
Deistung A, Schweser F, Reichenbach JR (2017) Overview of quantitative susceptibility mapping. NMR Biomed 30(4):e3569. https://doi.org/10.1002/nbm.3569
Wharton S, Bowtell R (2015) Effects of white matter microstructure on phase and susceptibility maps. Magn Reson Med 73(3):1258–1269. https://doi.org/10.1002/mrm.25189
Yablonskiy DA, Sukstanskii AL (2015) Generalized Lorentzian Tensor Approach (GLTA) as a biophysical background for quantitative susceptibility mapping. Magn Reson Med 73(2):757–764. https://doi.org/10.1002/mrm.25538
Li J, Chang S, Liu T, Wang Q, Cui D, Chen X, Jin M, Wang B, Pei M, Wisnieff C, Spincemaille P, Zhang M, Wang Y (2012) Reducing the object orientation dependence of susceptibility effects in gradient echo MRI through quantitative susceptibility mapping. Magn Reson Med 68(5):1563–1569. https://doi.org/10.1002/mrm.24135
Luo S, Yang L, Wang L (2015) Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. J Neuroradiol 42(5):255–260. https://doi.org/10.1016/j.neurad.2014.07.002
Wharton S, Bowtell R (2012) Fiber orientation-dependent white matter contrast in gradient echo MRI. Proc Natl Acad Sci 109(45):18559–18564. https://doi.org/10.1073/pnas.1211075109
Yablonskiy DA, Sukstanskii AL (2014) Biophysical mechanisms of myelin-induced water frequency shifts. Magn Reson Med 71(6):1956–1958. https://doi.org/10.1002/mrm.25214
Acosta-Cabronero J, Milovic C, Mattern H, Tejos C, Speck O, Callaghan MF (2018) A robust multi-scale approach to quantitative susceptibility mapping. NeuroImage 183:7–24. https://doi.org/10.1016/j.neuroimage.2018.07.065
Lim IAL, Faria AV, Li X, Hsu JTC, Airan RD, Mori S, van Zijl PCM (2013) Human brain atlas for automated region of interest selection in quantitative susceptibility mapping: application to determine iron content in deep gray matter structures. NeuroImage 82:449–469. https://doi.org/10.1016/j.neuroimage.2013.05.127
Langkammer C, Pirpamer L, Seiler S, Deistung A, Schweser F, Franthal S, Homayoon N, Katschnig-Winter P, Koegl-Wallner M, Pendl T, Stoegerer EM, Wenzel K, Fazekas F, Ropele S, Reichenbach JR, Schmidt R, Schwingenschuh P (2016) Quantitative susceptibility mapping in Parkinson’s disease. PLoS One 11(9):e0162460. https://doi.org/10.1371/journal.pone.0162460
Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR (2013) Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. NeuroImage 65:299–314. https://doi.org/10.1016/j.neuroimage.2012.09.055
Geyer S, Weiss M, Reimann K, Lohmann G, Turner R (2011) Microstructural parcellation of the human cerebral cortex – from Brodmann’s post-mortem map to in vivo mapping with high-field magnetic resonance imaging. Front Hum Neurosci 5:19. https://doi.org/10.3389/fnhum.2011.00019
Langkammer C, Liu T, Khalil M, Enzinger C, Jehna M, Fuchs S, Fazekas F, Wang Y, Ropele S (2013) Quantitative susceptibility mapping in multiple sclerosis. Radiology 267(2):551–559. https://doi.org/10.1148/radiol.12120707
Lebel RM, Eissa A, Seres P, Blevins G, Wilman AH (2012) Quantitative high-field imaging of sub-cortical gray matter in multiple sclerosis. Mult Scler J 18(4):433–441. https://doi.org/10.1177/1352458511428464
Walsh AJ, Lebel RM, Eissa A, Blevins G, Catz I, Lu J-Q, Resch L, Johnson ES, Emery DJ, Warren KG, Wilman AH (2013) Multiple sclerosis: validation of MR imaging for quantification and detection of iron. Radiology 267(2):531–542. https://doi.org/10.1148/radiol.12120863
Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D (2015) Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: interpreting positive susceptibility and the presence of iron. Magn Reson Med 74(2):564–570. https://doi.org/10.1002/mrm.25420
Taege Y, Hagemeier J, Bergsland N, Dwyer MG, Weinstock-Guttman B, Zivadinov R, Schweser F (2019) Assessment of mesoscopic properties of deep gray matter iron through a model-based simultaneous analysis of magnetic susceptibility and R2*—a pilot study in patients with multiple sclerosis and normal controls. NeuroImage 186:308–320. https://doi.org/10.1016/j.neuroimage.2018.11.011
Harrison DM, Li X, Liu H, Jones CK, Caffo B, Calabresi PA, van Zijl P (2016) Lesion heterogeneity on high-field susceptibility MRI is associated with multiple sclerosis severity. Am J Neuroradiol 37(8):1447–1453. https://doi.org/10.3174/ajnr.A4726
Li X, Harrison DM, Liu H, Jones CK, Oh J, Calabresi PA, van Zijl PCM (2016) Magnetic susceptibility contrast variations in multiple sclerosis lesions. J Magn Reson Imaging 43(2):463–473. https://doi.org/10.1002/jmri.24976
Pohmann R, Speck O, Scheffler K (2016) Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 Tesla using current receive coil arrays. Magn Reson Med 75(2):801–809. https://doi.org/10.1002/mrm.25677
Berry AS, Shah VD, Furman DJ, White RL III, Baker SL, O’Neil JP, Janabi M, D’Esposito M, Jagust WJ (2018) Dopamine synthesis capacity is associated with D2/3 receptor binding but not dopamine release. Neuropsychopharmacology 43(6):1201–1211. https://doi.org/10.1038/npp.2017.180
Treit S, Naji N, Seres P, Rickard J, Stolz E, Wilman AH, Beaulieu C (2021) R2* and quantitative susceptibility mapping in deep gray matter of 498 healthy controls from 5 to 90 years. Hum Brain Mapp 42(14):4597–4610. https://doi.org/10.1002/hbm.25569
Lozoff B, Georgieff MK (2006) Iron deficiency and brain development. Semin Pediatr Neurol 13(3):158–165. https://doi.org/10.1016/j.spen.2006.08.004
Grantham-McGregor S, Ani C (2001) A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr 131(2):649S–668S. https://doi.org/10.1093/jn/131.2.649S
McCann JC, Ames BN (2007) An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr 85(4):931–945. https://doi.org/10.1093/ajcn/85.4.931
Bodnar LM, Wisner KL (2005) Nutrition and depression: implications for improving mental health among childbearing-aged women. Biol Psychiatry 58(9):679–685. https://doi.org/10.1016/j.biopsych.2005.05.009
Corapci F, Calatroni A, Kaciroti N, Jimenez E, Lozoff B (2010) Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. J Pediatr Psychol 35(3):296–305. https://doi.org/10.1093/jpepsy/jsp065
Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS (2008) Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry 65(10):1136–1144. https://doi.org/10.1001/archpsyc.65.10.1136
Kim S-W, Stewart R, Park W-Y, Jhon M, Lee J-Y, Kim S-Y, Kim J-M, Amminger P, Chung Y-C, Yoon J-S (2018) Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients 10(11):1707. https://doi.org/10.3390/nu10111707
Konofal E, Lecendreux M, Arnulf I, Mouren M-C (2004) Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 158(12):1113. https://doi.org/10.1001/archpedi.158.12.1113
Lee H-S, Chao H-H, Huang W-T, Chen SC-C, Yang H-Y (2020) Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry 20(1):216. https://doi.org/10.1186/s12888-020-02621-0
Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW (2000) Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 105(4):e51. https://doi.org/10.1542/peds.105.4.e51
Oner O, Alkar OY, Oner P (2008) Relation of ferritin levels with symptom ratings and cognitive performance in children with attention deficit–hyperactivity disorder. Pediatr Int 50(1):40–44. https://doi.org/10.1111/j.1442-200X.2007.02496.x
Tseng P-T, Cheng Y-S, Chen Y-W, Stubbs B, Whiteley P, Carvalho AF, Li D-J, Chen T-Y, Yang W-C, Tang C-H, Chu C-S, Yang W-C, Liang H-Y, Wu C-K, Yen C-F, Lin P-Y (2018) Peripheral iron levels in children with autism spectrum disorders vs controls: a systematic review and meta-analysis. Nutr Res 50:44–52. https://doi.org/10.1016/j.nutres.2017.11.004
Tseng P-T, Cheng Y-S, Yen C-F, Chen Y-W, Stubbs B, Whiteley P, Carvalho AF, Li D-J, Chen T-Y, Yang W-C, Tang C-H, Chu C-S, Yang W-C, Liang H-Y, Wu C-K, Lin P-Y (2018) Peripheral iron levels in children with attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Sci Rep 8(1):788. https://doi.org/10.1038/s41598-017-19096-x
Yao S, Zhong Y, Xu Y, Qin J, Zhang N, Zhu X, Li Y (2017) Quantitative susceptibility mapping reveals an association between brain iron load and depression severity. Front Hum Neurosci 11:442. https://doi.org/10.3389/fnhum.2017.00442
Daugherty AM, Raz N (2015) Appraising the role of iron in brain aging and cognition: promises and limitations of MRI methods. Neuropsychol Rev 25(3):272–287. https://doi.org/10.1007/s11065-015-9292-y
Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S (2007) Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res 32(10):1655–1664. https://doi.org/10.1007/s11064-007-9352-7
Ghadery C, Pirpamer L, Hofer E, Langkammer C, Petrovic K, Loitfelder M, Schwingenschuh P, Seiler S, Duering M, Jouvent E, Schmidt H, Fazekas F, Mangin J-F, Chabriat H, Dichgans M, Ropele S, Schmidt R (2015) R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol Aging 36(2):925–932. https://doi.org/10.1016/j.neurobiolaging.2014.09.013
Li K, Reichmann H (2016) Role of iron in neurodegenerative diseases. J Neural Transm 123(4):389–399. https://doi.org/10.1007/s00702-016-1508-7
Núñez MT, Urrutia P, Mena N, Aguirre P, Tapia V, Salazar J (2012) Iron toxicity in neurodegeneration. Biometals 25(4):761–776. https://doi.org/10.1007/s10534-012-9523-0
Penke L, Valdés Hernandéz MC, Maniega SM, Gow AJ, Murray C, Starr JM, Bastin ME, Deary IJ, Wardlaw JM (2012) Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol Aging 33(3):510–517.e2. https://doi.org/10.1016/j.neurobiolaging.2010.04.032
Pujol J, Junqué C, Vendrell P, Grau JM, Martí-Vilalta JL, Olivé C, Gili J (1992) Biological significance of iron-related magnetic resonance imaging changes in the brain. Arch Neurol 49(7):711–717. https://doi.org/10.1001/archneur.1992.00530310053012
Arnsten AFT, Goldman-Rakic PS (1998) Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry 55(4):362–368. https://doi.org/10.1001/archpsyc.55.4.362
Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine Dl receptors in prefrontal cortex. Nature 376(6541):572–575. https://doi.org/10.1038/376572a0
Rodrigue KM, Daugherty AM, Foster CM, Kennedy KM (2020) Striatal iron content is linked to reduced fronto-striatal brain function under working memory load. NeuroImage 210:116544. https://doi.org/10.1016/j.neuroimage.2020.116544
Kalpouzos G, Garzón B, Sitnikov R, Heiland C, Salami A, Persson J, Bäckman L (2017) Higher striatal iron concentration is linked to frontostriatal underactivation and poorer memory in normal aging. Cereb Cortex 27(6):3427–3436. https://doi.org/10.1093/cercor/bhx045
Persson J, Garzón B, Sitnikov R, Bäckman L, Kalpouzos G (2020) A positive influence of basal ganglia iron concentration on implicit sequence learning. Brain Struct Funct 225(2):735–749. https://doi.org/10.1007/s00429-020-02032-7
Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E (1995) The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15(7):4851–4867. https://doi.org/10.1523/JNEUROSCI.15-07-04851.1995
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1):4–26. https://doi.org/10.1038/npp.2009.129
Darki F, Nemmi F, Möller A, Sitnikov R, Klingberg T (2016) Quantitative susceptibility mapping of striatum in children and adults, and its association with working memory performance. NeuroImage 136:208–214. https://doi.org/10.1016/j.neuroimage.2016.04.065
Carpenter KLH, Li W, Wei H, Wu B, Xiao X, Liu C, Worley G, Egger HL (2016) Magnetic susceptibility of brain iron is associated with childhood spatial IQ. NeuroImage 132:167–174. https://doi.org/10.1016/j.neuroimage.2016.02.028
Steiger TK, Weiskopf N, Bunzeck N (2016) Iron level and myelin content in the ventral striatum predict memory performance in the aging brain. J Neurosci 36(12):3552–3558. https://doi.org/10.1523/JNEUROSCI.3617-15.2016
Daugherty AM, Haacke EM, Raz N (2015) Striatal iron content predicts its shrinkage and changes in verbal working memory after two years in healthy adults. J Neurosci 35(17):6731–6743. https://doi.org/10.1523/JNEUROSCI.4717-14.2015
Biel D, Steiger TK, Bunzeck N (2021) Age-related iron accumulation and demyelination in the basal ganglia are closely related to verbal memory and executive functioning. Sci Rep 11(1):9438. https://doi.org/10.1038/s41598-021-88840-1
Bartzokis G, Lu PH, Tingus K, Peters DG, Amar CP, Tishler TA, Finn JP, Villablanca P, Altshuler LL, Mintz J, Neely E, Connor JR (2011) Gender and iron genes may modify associations between brain iron and memory in healthy aging. Neuropsychopharmacology 36(7):1375–1384. https://doi.org/10.1038/npp.2011.22
Sullivan EV, Adalsteinsson E, Rohlfing T, Pfefferbaum A (2009) Relevance of iron deposition in deep gray matter brain structures to cognitive and motor performance in healthy elderly men and women: exploratory findings. Brain Imaging Behav 3(2):167–175. https://doi.org/10.1007/s11682-008-9059-7
Valdés Hernández MdC, Ritchie S, Glatz A, Allerhand M, Muñoz Maniega S, Gow AJ, Royle NA, Bastin ME, Starr JM, Deary IJ, Wardlaw JM (2015) Brain iron deposits and lifespan cognitive ability. Age 37(5):100. https://doi.org/10.1007/s11357-015-9837-2
Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL (2007) An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics 120(2):e336–e345. https://doi.org/10.1542/peds.2006-2525
Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, Lozoff B, Jacobson SW (2010) Iron deficiency anemia and cognitive function in infancy. Pediatrics 126(2):e427–e434. https://doi.org/10.1542/peds.2009-2097
Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, Jimenez R, Mora LA, Gomez I, Krauskoph D (1987) Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics 79(6):981–995
Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME (1998) Behavior of infants with iron-deficiency anemia. Child Dev 69(1):24–36. https://doi.org/10.1111/j.1467-8624.1998.tb06130.x
Deinard AS, List A, Lindgren B, Hunt JV, Chang P-N (1986) Cognitive deficits in iron-deficient and iron-deficient anemic children. J Pediatr 108(5, Part 1):681–689. https://doi.org/10.1016/S0022-3476(86)81041-1
Pollitt E, Leibel RL, Greenfield DB (1983) Iron deficiency and cognitive test performance in preschool children. Nutr Behav 1(2):137–146
Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG (2001) Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics 107(6):1381–1386. https://doi.org/10.1542/peds.107.6.1381
Pollitt E, Hathiral P, Kotchabhakdi NJ, Missell L, Valyasevi A (1989) Iron deficiency and educational achievement in Thailand. Am J Clin Nutr 50(3):687–697. https://doi.org/10.1093/ajcn/50.3.687
Scott SP, De Souza MJ, Koehler K, Murray-Kolb LE (2017) Combined iron deficiency and low aerobic fitness doubly burden academic performance among women attending university. J Nutr 147(1):104–109. https://doi.org/10.3945/jn.116.240192
Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B (2010) Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci 13(2):54–70. https://doi.org/10.1179/147683010X12611460763689
Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T (2006) Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64(suppl_2):S34–S43. https://doi.org/10.1111/j.1753-4887.2006.tb00243.x
Wenger MJ, DellaValle DM, Murray-Kolb LE, Haas JD (2019) Effect of iron deficiency on simultaneous measures of behavior, brain activity, and energy expenditure in the performance of a cognitive task. Nutr Neurosci 22(3):196–206. https://doi.org/10.1080/1028415X.2017.1360559
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Parr, A., Larsen, B., Calabro, F., Tervo-Clemmens, B., Luna, B. (2023). Neuroimaging Human Dopamine-Related Neurophysiology Across Development. In: Fuentealba-Evans, J.A., Henny, P. (eds) Dopaminergic System Function and Dysfunction: Experimental Approaches. Neuromethods, vol 193. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2799-0_13
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2799-0_13
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2798-3
Online ISBN: 978-1-0716-2799-0
eBook Packages: Springer Protocols