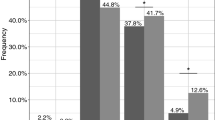
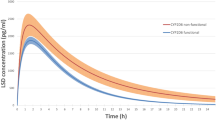
Abstract
ADHD is a common condition in both children and adults. The most prescribed medications for the treatment of ADHD include methylphenidate, mixed amphetamine salts, atomoxetine, guanfacine, and clonidine. While each of these medications have their own distinct pharmacokinetic profile, the extent to which pharmacogenetics effects their pharmacokinetic parameters is best described in atomoxetine, followed by methylphenidate. Atomoxetine is predominantly metabolized by cytochrome p450 2D6 (CYP2D6), while methylphenidate is metabolized by carboxylesterase 1 (CES1). Both CYP2D6 and CES1 have multiple variants resulting in varying levels of enzyme activity; however, to date, the functional consequence of variants and alleles for CYP2D6 is better characterized as compared to CES1. Regarding CYP2D6, individuals who are poor metabolizers prescribed atomoxetine experience up to ten-fold higher exposure as compared to normal metabolizers at comparable dosing. Additionally, individuals prescribed methylphenidate with the rs71647871 variant may experience up to 2.5-fold higher exposure as compared to those without. Having this pharmacogenetic information available may aid clinicians and patients when choosing medications and doses to treat ADHD.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Volkow ND, Swanson JM (2013) Clinical practice: adult attention deficit-hyperactivity disorder. N Engl J Med 369:1935–1944
Posner J, Polanczyk GV, Sonuga-Barke E (2020) Attention-deficit hyperactivity disorder. Lancet Lond Engl 395:450–462
Stevens T, Sangkuhl K, Brown JT et al (2019) PharmGKB summary: methylphenidate pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet Genomics 29:136–154
Elsayed NA, Yamamoto KM, Froehlich TE (2020) Genetic influence on efficacy of pharmacotherapy for pediatric attention-deficit/hyperactivity disorder: overview and current status of research. CNS Drugs 34:389–414
Brown JT, Bishop JR, Sangkuhl K et al (2019) Clinical pharmacogenetics implementation consortium guideline for cytochrome P450 (CYP)2D6 genotype and atomoxetine therapy. Clin Pharmacol Ther 106:94–102
Markowitz JS, Straughn AB, Patrick KS (2003) Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy 23:1281–1299
Merali Z, Ross S, Paré G (2014) The pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effect. Drug Metabol Drug Interact 29:143–151
Her L, Zhu H-J (2020) Carboxylesterase 1 and precision pharmacotherapy: pharmacogenetics and nongenetic regulators. Drug Metab Dispos Biol Fate Chem 48:230–244
Wang X, Rida N, Shi J et al (2017) A comprehensive functional assessment of carboxylesterase 1 nonsynonymous polymorphisms. Drug Metab Dispos Biol Fate Chem 45:1149–1155
Zhu H-J, Patrick KS, Yuan H-J et al (2008) Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet 82:1241–1248
Tarkiainen EK, Backman JT, Neuvonen M et al (2012) Carboxylesterase 1 polymorphism impairs oseltamivir bioactivation in humans. Clin Pharmacol Ther 92:68–71
Tarkiainen EK, Tornio A, Holmberg MT et al (2015) Effect of carboxylesterase 1 c.428G >A single nucleotide variation on the pharmacokinetics of quinapril and enalapril. Br J Clin Pharmacol 80:1131–1138
Lewis JP, Horenstein RB, Ryan K et al (2013) The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics 23:1–8
Paré G, Eriksson N, Lehr T et al (2013) Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation 127:1404–1412
Stage C, Jürgens G, Guski LS et al (2017) The impact of CES1 genotypes on the pharmacokinetics of methylphenidate in healthy Danish subjects. Br J Clin Pharmacol 83:1506–1514
Lyauk YK, Stage C, Bergmann TK et al (2016) Population pharmacokinetics of methylphenidate in healthy adults emphasizing novel and known effects of several carboxylesterase 1 (CES1) variants. Clin Transl Sci 9:337–345
Stage C, Dalhoff K, Rasmussen HB et al (2019) The impact of human CES1 genetic variation on enzyme activity assessed by ritalinic acid/methylphenidate ratios. Basic Clin Pharmacol Toxicol 125:54–61
ClinicalTrials.gov [Internet]. [cited 15 Sep 2022]. Available from: https://clinicaltrials.gov/
Brown JT, Bishop JR (2015) Atomoxetine pharmacogenetics: associations with pharmacokinetics, treatment response and tolerability. Pharmacogenomics 16:1513–1520
Strattera [Package insert]. Eli Lilly and Company, Indianapolis
Swen JJ, Nijenhuis M, de Boer A et al (2011) Pharmacogenetics: from bench to byte – an update of guidelines. Clin Pharmacol Ther 89:662–673
Zerbe RL, Rowe H, Enas GG et al (1985) Clinical pharmacology of tomoxetine, a potential antidepressant. J Pharmacol Exp Ther 232:139–143
Farid NA, Bergstrom RF, Ziege EA et al (1985) Single-dose and steady-state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol 25:296–301
Sauer J-M, Ponsler GD, Mattiuz EL et al (2003) Disposition and metabolic fate of atomoxetine hydrochloride: the role of CYP2D6 in human disposition and metabolism. Drug Metab Dispos Biol Fate Chem 31:98–107
Witcher JW, Long A, Smith B et al (2003) Atomoxetine pharmacokinetics in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 13:53–63
Brown JT, Abdel-Rahman SM, van Haandel L et al (2016) Single dose, CYP2D6 genotype-stratified pharmacokinetic study of atomoxetine in children with ADHD. Clin Pharmacol Ther 99:642–650
Cui YM, Teng CH, Pan AX et al (2007) Atomoxetine pharmacokinetics in healthy Chinese subjects and effect of the CYP2D6*10 allele. Br J Clin Pharmacol 64:445–449
Matsui A, Azuma J, Witcher JW et al (2012) Pharmacokinetics, safety, and tolerability of atomoxetine and effect of CYP2D6*10/*10 genotype in healthy Japanese men. J Clin Pharmacol 52:388–403
Byeon J-Y, Kim Y-H, Na H-S et al (2015) Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharm Res 38:2083–2091
Bradford LD (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 3:229–243
Sistonen J, Sajantila A, Lao O et al (2007) CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics 17:93–101
Adderall XR [Package insert]. Shire LLC, Wayne
Claessens AJ, Risler LJ, Eyal S et al (2010) CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos Biol Fate Chem 38:1393–1396
Table of Pharmacogenetic Associations [Internet]. [cited 15 Sep 2021]. Available from: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations
Li X-Y, Hu X-X, Yang F et al (2019) Effects of 24 CYP2D6 variants found in Chinese population on the metabolism of clonidine in vitro. Chem Biol Interact 313:108840
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Brown, J.T. (2022). The Pharmacogenetic Impact on the Pharmacokinetics of ADHD Medications. In: Yan, Q. (eds) Pharmacogenomics in Drug Discovery and Development. Methods in Molecular Biology, vol 2547. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2573-6_15
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2573-6_15
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2572-9
Online ISBN: 978-1-0716-2573-6
eBook Packages: Springer Protocols