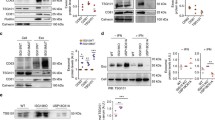
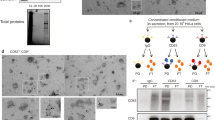
Abstract
Cells use unconventional secretion to deliver the β-galactoside binding lectin galectin-3 from the cell interior into the extracellular milieu. This process starts with galectin-3 recruitment into intraluminal vesicles (ILVs), which are later released at the plasma membrane as exosomes. Electron microscopy is utilized to determine the location of GFP-tagged galectin-3 in pelleted exosomes. We also describe how these vesicles are harvested from cell culture media to determine their composition. The fluorescent protein GFP was fused with the exosomal sorting motif of galectin-3 to direct GFP into exosomes. Recruitment of this fusion construct into the lumen of exosomes can be assessed by proteinase K accessibility analysis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rabouille C (2017) Pathways of unconventional protein secretion. Trends Cell Biol 27(3):230–240. https://doi.org/10.1016/j.tcb.2016.11.007
Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F (2004) Introduction to galectins. Glycoconj J 19(7–9):433–440
Lepur A, Salomonsson E, Nilsson UJ, Leffler H (2012) Ligand induced galectin-3 protein self-association. J Biol Chem 287(26):21751–21756. https://doi.org/10.1074/jbc.C112.358002. C112.358002 [pii]
Bänfer S, Schneider D, Dewes J, Strauss MT, Freibert S-A, Heimerl T, Maier UG, Elsässer H-P, Jungmann R, Jacob R (2018) Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc Natl Acad Sci 115(19):E4396–E4405. https://doi.org/10.1073/pnas.1718921115
Fortuna-Costa A, Gomes AM, Kozlowski EO, Stelling MP, Pavão MSG (2014) Extracellular galectin-3 in tumor progression and metastasis. Front Oncol 4:138–138. https://doi.org/10.3389/fonc.2014.00138
Johannes L, Jacob R, Leffler H (2018) Galectins at a glance. J Cell Sci 131(9). https://doi.org/10.1242/jcs.208884
Delacour D, Jacob R (2006) Apical protein transport. Cell Mol Life Sci 63(21):2491–2505
Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R (2006) Requirement for galectin-3 in apical protein sorting. Curr Biol 16(4):408–414
Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H (1993) Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem 268(16):11750–11757
Sato S, Burdett I, Hughes RC (1993) Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res 207(1):8–18. https://doi.org/10.1006/excr.1993.1157. S0014-4827(83)71157-2 [pii]
Cleves AE, Cooper DN, Barondes SH, Kelly RB (1996) A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol 133(5):1017–1026. https://doi.org/10.1083/jcb.133.5.1017
Hughes RC (1999) Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta 1473(1):172–185
Stalz H, Roth U, Schleuder D, Macht M, Haebel S, Strupat K, Peter-Katalinic J, Hanisch FG (2006) The Geodia cydonium galectin exhibits prototype and chimera-type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology 16(5):402–414. https://doi.org/10.1093/glycob/cwj086
Muller WE, Conrad J, Schroder C, Zahn RK, Kurelec B, Dreesbach K, Uhlenbruck G (1983) Characterization of the trimeric, self-recognizing Geodia cydonium lectin I. Eur J Biochem 133(2):263–267
Wagner-Hülsmann C, Bachinski N, Diehl-Seifert B, Blumbach B, Steffen R, Pancer Z, Müller WEG (1996) A galectin links the aggregation factor to cells in the sponge (Geodia cydonium) system. Glycobiology 6(8):785–793. https://doi.org/10.1093/glycob/6.8.785-d
Fan X, She Y-M, Bagshaw RD, Callahan JW, Schachter H, Mahuran DJ (2005) Identification of the hydrophobic glycoproteins of Caenorhabditis elegans. Glycobiology 15(10):952–964. https://doi.org/10.1093/glycob/cwi075
Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA (2011) When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50(37):7842–7857. https://doi.org/10.1021/bi201121m
Ideo H, Fukushima K, Gengyo-Ando K, Mitani S, Dejima K, Nomura K, Yamashita K (2009) A Caenorhabditis elegans glycolipid-binding galectin functions in host defense against bacterial infection. J Biol Chem 284(39):26493–26501. https://doi.org/10.1074/jbc.M109.038257
Rabouille C, Malhotra V, Nickel W (2012) Diversity in unconventional protein secretion. J Cell Sci 125(22):5251–5255. https://doi.org/10.1242/jcs.103630
Nickel W, Rabouille C (2009) Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 10(2):148–155. https://doi.org/10.1038/nrm2617
Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, Benito-Martin A, Burillo E, Zalba G, Beloqui O, Llamas-Granda P, Ortiz A, Egido J, Blanco-Colio LM, Martin-Ventura JL (2014) Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc 3(4):e000785. https://doi.org/10.1161/jaha.114.000785
Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA (2009) Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20(2):363–379. https://doi.org/10.1681/ASN.2008040406
Liang B, Peng P, Chen S, Li L, Zhang M, Cao D, Yang J, Li H, Gui T, Li X, Shen K (2013) Characterization and proteomic analysis of ovarian cancer-derived exosomes. J Proteome 80:171–182. https://doi.org/10.1016/j.jprot.2012.12.029
Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD, Clayton A (2010) Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics 9(6):1324–1338. https://doi.org/10.1074/mcp.M000063-MCP201
Pornillos O, Alam SL, Rich RL, Myszka DG, Davis DR, Sundquist WI (2002) Structure and functional interactions of the Tsg101 UEV domain. EMBO J 21(10):2397–2406. https://doi.org/10.1093/emboj/21.10.2397
Richardson JC, Scalera V, Simmons NL (1981) Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta 673(1):26–36
Delacour D, Greb C, Koch A, Salomonsson E, Leffler H, Le Bivic A, Jacob R (2007) Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic 8(4):379–388
Savina A, Furlan M, Vidal M, Colombo MI (2003) Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 278(22):20083–20090. https://doi.org/10.1074/jbc.M301642200
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183(3):1161–1172. https://doi.org/10.1084/jem.183.3.1161
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212. https://doi.org/10.1083/jcb.17.1.208
Kalra H, Adda CG, Liem M, Ang C-S, Mechler A, Simpson RJ, Hulett MD, Mathivanan S (2013) Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 13(22):3354–3364. https://doi.org/10.1002/pmic.201300282
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3:Unit 3.22. https://doi.org/10.1002/0471143030.cb0322s30
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Bänfer, S., Kutscher, S., Jacob, R. (2022). Examination of Galectin-3 Recruitment into Multivesicular Bodies for Exosomal Secretion. In: Stowell, S.R., Arthur, C.M., Cummings, R.D. (eds) Galectins. Methods in Molecular Biology, vol 2442. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2055-7_22
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2055-7_22
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2054-0
Online ISBN: 978-1-0716-2055-7
eBook Packages: Springer Protocols