Abstract
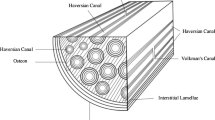
A multiscale modeling technique was developed to predict mechanical properties of human bone, which utilizes the hierarchies of human bone in different length scales from nanoscale to macroscale. Bone has a unique structure displaying high stiffness with minimal weight. This is achieved through a hierarchy of complex geometries composed of three major materials: hydroxyapatite, collagen and water. The identifiable hierarchical structures of bone are hydroxyapatite, tropocollagen, fibril, fiber, lamellar layer, trabecular bone, cancellous bone and cortical bone. A helical spring model was used to represent the stiffness of collagen. A unit cell-based micromechanics model computed both the normal and shear stiffness of fibrils, fibers, and lamellar layers. A laminated composite model was applied to cortical and trabecular bone, while a simplified finite element model for the tetrakaidecahedral shape was used to evaluate cancellous bone. Modeling bone from nanoscale components to macroscale structures allows the influence of each structure to be assessed. It was found that the distribution of hydroxyapatite within the tropocollagen matrix at the fibril level influences the macroscale properties significantly. Additionally, the multiscale analysis model can vary any parameter of any hierarchical level to determine its effect on the bone property. With so little known about the detailed structure of nanoscale and microscale bone, a model encompassing the complete hierarchy of bone can be used to help validate assumptions or hypotheses about those structures.







Similar content being viewed by others
References
Ashman RB, Rho JY (1988) Elastic modulus of trabecular bone material. J Biomech 21(3):177–181
Buehler MJ (2006) Atomistic and continuum modeling of mechanical properties of collagen: elasticity, fracture, and self-assembly. J Mater Res 21(8):1947–1961
Buschow KH, Cahn J, Flemings RW, Ilschner MC, Kramer B, Mahajan EJ (2001) Bone mineralization. In: Encyclopedia of materials science and technology, pp 787–794
Carter DR, Caler WE (1981) Uniaxial fatigue of human cortical bone. The influence of tissue physical characteristics. J Biomech 14(7):461–470
Cowin SC, Mehrabadi MM (1989) Identification of the elastic symmetry of bone and other materials. J Biomech 22(6–7):503–515
Cui FZ, Li Y, Ge J (2007) Self-assembly of mineralized collagen composites. Mater Sci Eng 57:1–27
Ding M, Hvid I (2000) Quantification of age-related changes in the structure model type and trabecular thickness of human tibial cancellous bone. Bone 26(3):291–295
Fyhrie DP, Schaffler MB (1994) Failure mechanisms in human vertebral cancellous bone. Bone 15(1):105–109
Gautieri A, Vesentini S, Redaelli A, Ballarini R (2013) Modeling and measuring visco-elastic properties: From collagen molecules to collagen fibrils. Int J Non-Linear Mech 56:25–33
Gibson LJ (1985) The mechanical behavior of cancellous bone. J Biomech 18(5):317–328
Gibson RF (1994) Principles of composite material mechanics. McGraw-Hill Inc., New York
Gong H, Zhu D, Gao J, Linwei L, Zhang X (2010) Ad adaptation model for trabecular bone at different mechanical levels. BioMed Eng OnLine 9(32):1–17
Guo XE, Kim CH (2002) Mechanical consequence of trabecular bone loss and its treatment: a three-dimensional model simulation. Bone 30(2):404–411
Hamed E, Jasiuk I (2013) Multiscale damage and strength of lamellar bone modeled by cohesive finite elements. J Mech Behav Biomed Mater 28:94–110
Harley R, James D, Miller A, White JW (1997) Phonons and the elastic moduli of collagen and muscle. Nature 267:285–287
Hench LL, Jones JR (2005) Clinical needs and concepts of repair, biomaterials. In: Hench LL, Jones JR (eds) Artificial organs and tissue eng. Sawston, UK, Woodhead, pp 79–89
Kadir MR, Syahrom A, Öchsner A (2010) Finite element analysis of idealised unit cell cancellous structure based on morphological indices of cancellous bone. Med Biol Eng Comput 48:497–505
Kotha SP, Guzelsu N (2007) Tensile behavior of cortical bone: dependence of organic matrix material properties on bone mineral content. J Biomech 40:36–45
Krug R, Carballido-Gamio J, Burhardt AJ, Kazakia G, Hyun BH, Jobke B, Banerjee S, Huber M, Link TM TM, Majumdar S (2008) Assessment of trabecular bone structure comparing magnetic resonance imaging at 3 Tesla with high-resolution peripheral quantitative computed tomography ex vivo and in vivo. Osteoporos Int 19(5):653–661
Kwon YW, Cooke RE, Park C (2003) Representative unit-cell models for open-cell metal foams with or without elastic fillers. Mater Sci Eng A 343:63–70
Kwon YW, Darcy J (2018) Failure criteria for fibrous composites based on multiscale model. Multiscale Multidiscip Model Exp Des 1(1):3–17
Kwon YW, Kim C (1998) Micromechanical model for thermal analysis of particulate and fibrous composites. J Therm Stress 21:21–39
Kwon YW, Park MS (2013) Versatile micromechanics model for multiscale analysis of composite structures. Appl Compos Mater 20(4):673–692
Nikolaeva TI, Tiktopulo EI, Il’yasova EN, Kuznetsova SM (2007) Collagen type I fibril packing in vivo and in vitro. Biophysics 52(5):489–497
Majumar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC, Mathur A (1997) Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 12(1):111–118
Odgaard A (1997) Three-dimensional methods for quantification of cancellous bone architecture. Bone 20(4):315–328
Park MS, Kwon YW (2013) Elastoplastic micromechanics model for multiscale analysis of metal matrix composite structures. Comput Struct 123:28–38
Parkinson IH, Fazzalari NL (2013) Characterisation of trabecular bone, studies in mechanobiology, tissue engineering and biomaterials, vol 5, pp 31–51
Reznikov N, Shahar R, Weiner S (2014) Bone hierarchical structure in three dimensions. Acta Biomater 10:3815–3826
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20:92–102
Ricard-Blum S (2011) The collagen family. Cold Spring Harb Perspect Biol 3(1):a004978
Skedros JG, Holmes JL, Vajda EG, Bloebaum RD (2005) Cement lines of secondary osteons in human bone are not mineral-deficient: New data in a historical perspective. Anat Rec Part 286A(1):781–803
Tzaphlidou M, Berillis P (2005) Collagen fibril diameter in relation to bone site. A quantitative ultrastructural study. Micron 36:703–705
Vaughan TJ, McCarthy CT, McNamara LM (2012) A three-scale finite element investigation into the effects of tissue mineralisation and lamellar organisation in human cortical and trabecular bone. J Mech Behav Biomed Mater 12:50–62
Yoon HS, Katz JL (1976) Ultrasonic wave propagation in human cortical bone-II. Measurements of elastic properties and microhardness. J Biomech 9(7):459–464
Zamiri A, De S (2011) Mechanical properties of hydroxapatite single crystals from nanoindentation data. J Mech Behav Biomed Mater 2(4):146–152
Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA (1999) Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech 32(10):1005–1012
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwon, Y.W., Clumpner, B.R. Multiscale modeling of human bone. Multiscale and Multidiscip. Model. Exp. and Des. 1, 133–143 (2018). https://doi.org/10.1007/s41939-018-0013-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41939-018-0013-0