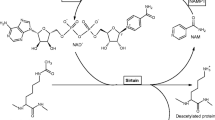
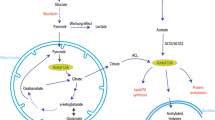
Abstract
The survival of an organism is intricately dependent upon its ability to sense and respond to both extracellular and intracellular cues. In the context of metabolic or nutrient sensing while intracellular signaling ensures synchronization of various metabolic pathways, inter-tissue communication enables the organism to couple energetic needs of all the organ systems and in a concerted manner. In this review, we highlight the role of evolutionarily conserved sirtuins (NAD-dependent deacylases) in synchronizing inter-organellar and inter-tissue cross-talk that is needed to orchestrate organism-wide metabolic homeostasis.


Similar content being viewed by others
References
Riera CE, Dillin A (2015) Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol 17:196–203. doi:10.1038/ncb3107
Roberts SB, Rosenberg I (2006) Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev 86:651–667. doi:10.1152/physrev.00019.2005
Bailey SM, Udoh US, Young ME (2014) Circadian regulation of metabolism. J Endocrinol 222:R75–R96. doi:10.1530/JOE-14-0200
Eckel-Mahan K, Sassone-Corsi P (2013) Metabolism and the circadian clock converge. Physiol Rev 93:107–135. doi:10.1152/physrev.00016.2012
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13:251–262. doi:10.1038/nrm3311
Tanner KG, Landry J, Sternglanz R, Denu JM (2000) Silent information regulator 2 family of NAD- dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci USA 97:14178–14182. doi:10.1073/pnas.250422697
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795–800. doi:10.1038/35001622
Schmelzle T, Hall MN (2000) TOR, a central controller of cell growth. Cell 103:253–262
Scarpulla RC, Vega RB, Kelly DP (2012) Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23:459–466. doi:10.1016/j.tem.2012.06.006
Hock MB, Kralli A (2009) Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71:177–203. doi:10.1146/annurev.physiol.010908.163119
Scarpulla RC (2002) Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286:81–89
Guha M, Avadhani NG (2013) Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion 13:577–591. doi:10.1016/j.mito.2013.08.007
Butow RA, Avadhani NG (2004) Mitochondrial signaling: the retrograde response. Mol Cell 14:1–15
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806. doi:10.1038/414799a
Cheatham B, Kahn CR (1995) Insulin action and the insulin signaling network. Endocr Rev 16:117–142. doi:10.1210/edrv-16-2-117
White MF (2003) Insulin signaling in health and disease. Science 302:1710–1711. doi:10.1126/science.1092952
Tatar M, Bartke A, Antebi A (2003) The endocrine regulation of aging by insulin-like signals. Science 299:1346–1351. doi:10.1126/science.1081447
Bartke A (2005) Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 146:3718–3723. doi:10.1210/en.2005-0411
Giannakou ME, Partridge L (2007) Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci 32:180–188. doi:10.1016/j.tibs.2007.02.007
Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277:942–946
Taguchi A, White MF (2008) Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol 70:191–212. doi:10.1146/annurev.physiol.70.113006.100533
Tatar M et al (2001) A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292:107–110. doi:10.1126/science.1057987
Gottlieb S, Esposito RE (1989) A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56:771–776
Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M (1997) SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev 11:83–93
Lee SE, Paques F, Sylvan J, Haber JE (1999) Role of yeast SIR genes and mating type in directing DNA double-strand breaks to homologous and non-homologous repair paths. Curr Biol 9:767–770
Banerjee KK et al (2012) dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep 2:1485–1491
Banerjee KK, Ayyub C, Sengupta S, Kolthur-Seetharam U (2013) Fat body dSir2 regulates muscle mitochondrial physiology and energy homeostasis nonautonomously and mimics the autonomous functions of dSir2 in muscles. Mol Cell Biol 33:252–264
Rogina B, Helfand SL (2004) Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA 101:15998–16003. doi:10.1073/pnas.0404184101
Tissenbaum HA, Guarente L (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410:227–230. doi:10.1038/35065638
Guarente L (2013) Calorie restriction and sirtuins revisited. Genes Dev 27:2072–2085. doi:10.1101/gad.227439.113
Haigis MC, Guarente LP (2006) Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 20:2913–2921. doi:10.1101/gad.1467506
Greiss S, Gartner A (2009) Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. Mol Cells 28:407–415. doi:10.1007/s10059-009-0169-x
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13. doi:10.1042/BJ20070140
Bheda P, Jing H, Wolberger C, Lin H (2016) The substrate specificity of sirtuins. Annu Rev Biochem 85:405–429. doi:10.1146/annurev-biochem-060815-014537
Ghosh S, George S, Roy U, Ramachandran D, Kolthur-Seetharam U (2010) NAD: a master regulator of transcription. Biochim Biophys Acta 1799:681–693. doi:10.1016/j.bbagrm.2010.08.002
Bonkowski MS, Sinclair DA (2016) Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. doi:10.1038/nrm.2016.93
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13:225–238. doi:10.1038/nrm3293
Longo VD, Kennedy BK (2006) Sirtuins in aging and age-related disease. Cell 126:257–268. doi:10.1016/j.cell.2006.07.002
Libert S et al (2011) SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 147:1459–1472. doi:10.1016/j.cell.2011.10.054
Pillai VB, Sundaresan NR, Gupta MP (2014) Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res 114:368–378. doi:10.1161/CIRCRESAHA.113.300536
Price NL et al (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. doi:10.1016/j.cmet.2012.04.003
Lagouge M et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122. doi:10.1016/j.cell.2006.11.013
Purushotham A et al (2009) Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9:327–338. doi:10.1016/j.cmet.2009.02.006
Gerhart-Hines Z et al (2007) Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26:1913–1923. doi:10.1038/sj.emboj.7601633
Wang RH, Li C, Deng CX (2010) Liver steatosis and increased ChREBP expression in mice carrying a liver specific SIRT1 null mutation under a normal feeding condition. Int J Biol Sci 6:682–690
Chen H et al (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495
Li Y et al (2011) Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J 25:1664–1679. doi:10.1096/fj.10-173492
Canto C et al (2009) AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature 458:1056–1060. doi:10.1038/nature07813
Banks AS et al (2008) SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8:333–341. doi:10.1016/j.cmet.2008.08.014
Liang F, Kume S, Koya D (2009) SIRT1 and insulin resistance. Nat Rev Endocrinol 5:367–373. doi:10.1038/nrendo.2009.101
Palu RA, Thummel CS (2016) Sir2 acts through hepatocyte nuclear factor 4 to maintain insulin signaling and metabolic homeostasis in Drosophila. PLoS Genet 12:e1005978. doi:10.1371/journal.pgen.1005978
Yoshizaki T et al (2009) SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29:1363–1374. doi:10.1128/MCB.00705-08
Zhang J (2007) The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem 282:34356–34364. doi:10.1074/jbc.M706644200
Schenk S et al (2011) Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121:4281–4288. doi:10.1172/JCI58554
Ikenoue T, Inoki K, Zhao B, Guan KL (2008) PTEN acetylation modulates its interaction with PDZ domain. Cancer Res 68:6908–6912. doi:10.1158/0008-5472.CAN-08-1107
Sun C et al (2007) SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319
Sundaresan NR et al (2011) The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal 4:ra46. doi:10.1126/scisignal.2001465
Chae HD, Broxmeyer HE (2011) SIRT1 deficiency downregulates PTEN/JNK/FOXO1 pathway to block reactive oxygen species-induced apoptosis in mouse embryonic stem cells. Stem Cells Dev 20:1277–1285. doi:10.1089/scd.2010.0465
Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L (2007) Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev 128:546–552. doi:10.1016/j.mad.2007.07.007
Bordone L et al (2006) Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 4:e31. doi:10.1371/journal.pbio.0040031
Moynihan KA et al (2005) Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2:105–117. doi:10.1016/j.cmet.2005.07.001
Ramachandran D et al (2011) Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic beta-islets. FEBS J 278:1167–1174. doi:10.1111/j.1742-4658.2011.08042.x
Monteserin-Garcia J et al (2013) Sirt1 inhibits the transcription factor CREB to regulate pituitary growth hormone synthesis. FASEB J 27:1561–1571. doi:10.1096/fj.12-220129
Yamamoto M et al (2013) SIRT1 regulates adaptive response of the growth hormone–insulin-like growth factor-I axis under fasting conditions in liver. Proc Natl Acad Sci USA 110:14948–14953. doi:10.1073/pnas.1220606110
Li Y et al (2014) Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology 146:539–549. doi:10.1053/j.gastro.2013.10.059
Hebert AS et al (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49:186–199. doi:10.1016/j.molcel.2012.10.024
Rardin MJ et al (2013) Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci USA 110:6601–6606. doi:10.1073/pnas.1302961110
Ahn BH et al (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105:14447–14452. doi:10.1073/pnas.0803790105
Vassilopoulos A et al (2014) SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid Redox Signal 21:551–564. doi:10.1089/ars.2013.5420
Yu W, Dittenhafer-Reed KE, Denu JM (2012) SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287:14078–14086. doi:10.1074/jbc.M112.355206
Cimen H et al (2010) Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49:304–311. doi:10.1021/bi901627u
Bharathi SS et al (2013) Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J Biol Chem 288:33837–33847. doi:10.1074/jbc.M113.510354
Hallows WC et al (2011) Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell 41:139–149. doi:10.1016/j.molcel.2011.01.002
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12:662–667. doi:10.1016/j.cmet.2010.11.015
Tao R et al (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40:893–904. doi:10.1016/j.molcel.2010.12.013
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A (2009) Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89:27–71. doi:10.1152/physrev.00014.2008
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15. doi:10.1083/jcb.201102095
Jing E et al (2011) Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA 108:14608–14613. doi:10.1073/pnas.1111308108
Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I (2005) Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 16:4623–4635
Haigis MC et al (2006) SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126:941–954
Jeong SM et al (2013) SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23:450–463. doi:10.1016/j.ccr.2013.02.024
Ho L et al (2013) SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging (Albany NY) 5:835–849. doi:10.18632/aging.100616
Laurent G et al (2013) SIRT4 represses peroxisome proliferator-activated receptor alpha activity to suppress hepatic fat oxidation. Mol Cell Biol 33:4552–4561. doi:10.1128/MCB.00087-13
Laurent G et al (2013) SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell 50:686–698. doi:10.1016/j.molcel.2013.05.012
Zhong L et al (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140:280–293. doi:10.1016/j.cell.2009.12.041
Kugel S, Mostoslavsky R (2014) Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci 39:72–81. doi:10.1016/j.tibs.2013.12.002
Xiao C et al (2010) SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem 285:36776–36784. doi:10.1074/jbc.M110.168039
Sundaresan NR et al (2012) The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med 18:1643–1650. doi:10.1038/nm.2961
Mostoslavsky R et al (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124:315–329
Eijkelenboom A, Burgering BM (2013) FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 14:83–97. doi:10.1038/nrm3507
Xiong X et al (2016) SIRT6 protects against palmitate-induced pancreatic beta-cell dysfunction and apoptosis. J Endocrinol. doi:10.1530/JOE-16-0317
Song MY, Wang J, Ka SO, Bae EJ, Park BH (2016) Insulin secretion impairment in Sirt6 knockout pancreatic beta cells is mediated by suppression of the FoxO1-Pdx1-Glut2 pathway. Sci Rep 6:30321. doi:10.1038/srep30321
Xiong X et al (2016) Sirtuin 6 regulates glucose-stimulated insulin secretion in mouse pancreatic beta cells. Diabetologia 59:151–160. doi:10.1007/s00125-015-3778-2
Kanfi Y et al (2012) The sirtuin SIRT6 regulates lifespan in male mice. Nature 483:218–221. doi:10.1038/nature10815
Jiang H, Zhang X, Lin H (2016) Lysine fatty acylation promotes lysosomal targeting of TNF-alpha. Sci Rep 6:24371. doi:10.1038/srep24371
Zhang X et al (2016) Identifying the functional contribution of the defatty-acylase activity of SIRT6. Nat Chem Biol 12:614–620. doi:10.1038/nchembio.2106
Burnett C et al (2011) Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477:482–485. doi:10.1038/nature10296
Hoffmann J, Romey R, Fink C, Yong L, Roeder T (2013) Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging (Albany NY) 5:315–327. doi:10.18632/aging.100553
Chang HC, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25:138–145. doi:10.1016/j.tem.2013.12.001
Giblin W, Skinner ME, Lombard DB (2014) Sirtuins: guardians of mammalian healthspan. Trends Genet 30:271–286. doi:10.1016/j.tig.2014.04.007
Vaquero A et al (2007) SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450:440–444
Zocchi L, Sassone-Corsi P (2012) SIRT1-mediated deacetylation of MeCP2 contributes to BDNF expression. Epigenetics 7:695–700. doi:10.4161/epi.20733
Wood JG et al (2016) Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc Natl Acad Sci USA 113:11277–11282. doi:10.1073/pnas.1604621113
Oberdoerffer P et al (2008) SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135:907–918. doi:10.1016/j.cell.2008.10.025
Tsurumi A, Li WX (2012) Global heterochromatin loss: a unifying theory of aging? Epigenetics 7:680–688. doi:10.4161/epi.20540
Gomes AP et al (2013) Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155:1624–1638. doi:10.1016/j.cell.2013.11.037
Ramadori G et al (2011) SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab 14:301–312. doi:10.1016/j.cmet.2011.06.014
Kim HS et al (2010) Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab 12:224–236. doi:10.1016/j.cmet.2010.06.009
Lee E et al (2012) Landscape of somatic retrotransposition in human cancers. Science 337:967–971. doi:10.1126/science.1222077
Reilly MT, Faulkner GJ, Dubnau J, Ponomarev I, Gage FH (2013) The role of transposable elements in health and diseases of the central nervous system. J Neurosci 33:17577–17586. doi:10.1523/JNEUROSCI.3369-13.2013
Van Meter M et al (2014) SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat Commun 5:5011. doi:10.1038/ncomms6011
Kawahara TL et al (2011) Dynamic chromatin localization of Sirt6 shapes stress- and aging-related transcriptional networks. PLoS Genet 7:e1002153. doi:10.1371/journal.pgen.1002153
Bao X, Wang Y, Li X, Li XM, Liu Z, Yang T, Wong CF, Zhang J, Hao Q, Li XD (2014) Identification of ‘erasers’ for lysine crotonylated histone marks using a chemical proteomics approach. Elife 3:e02999
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Molecular cell 11(2):437–444
Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP (2002) SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Nat Acad Sci 99(21):13653–13658
Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, Kang Y, Shenk T, Cristea IM (2014) Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159(7):1615-1625
Nakagawa T, Lomb DJ, Haigis MC, Guarente L (2009) SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137(3):560–570
Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B (2011) Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334(6057):806-809
Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, Ro J (2014) Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell metab 19(4):605–617
Liszt G, Ford E, Kurtev M, Guarente L (2005) Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem 280(22):21313–21320
Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY (2008) SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452(7186):492–496
Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, Struhl K (2012) SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 487(7405):114–118
Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, He L, Li W, Yi X, Sun L, Liang J (2016) SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun 7:12235 doi: 10.1038/ncomms12235
Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC (2013) SIRT6 regulates TNF-[agr] secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496(7443):110–113
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Deota and N. Shukla contributed equally.
Rights and permissions
About this article
Cite this article
Deota, S., Shukla, N. & Kolthur-Seetharam, U. Spatio-Temporal Control of Cellular and Organismal Physiology by Sirtuins. J Indian Inst Sci 97, 147–159 (2017). https://doi.org/10.1007/s41745-016-0018-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-016-0018-9