Abstract
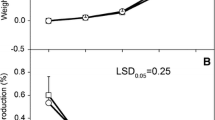
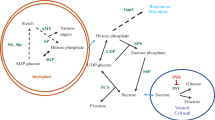
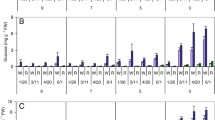
When stored at temperatures below 10 °C, potatoes accumulate sucrose and the reducing sugars glucose and fructose. This process, cold-induced sweetening, has been studied extensively because potatoes with elevated reducing sugar contents produce undesirable, dark-colored products and acrylamide, a suspected carcinogen, during high-temperature cooking. Potatoes in commercial storages are cooled slowly, but many research studies have used potatoes cooled rapidly. In this study, effects of cooling rate and variety on chip color, sugars, and gene expression were examined. Sucrose and reducing sugar contents were substantially lower in slowly cooled than in rapidly cooled tubers of ‘Snowden’ and “MegaChip’ for the first 11 weeks after cooling to 3 °C began. Differences in gene expression for VInv, β-amylase, SPS, AGPase and GBSS were observed between cooling treatments and varieties. Overall, the data showed that cooling rate, time in storage, and variety influenced multiple aspects of cold-induced sweetening.
Resumen
Cuando se almacena a temperaturas por debajo de los 10 °C, las papas acumulan sacarosa y los azúcares reductores glucosa y fructosa. Este proceso de endulzamiento inducido por el frío, se ha estudiado extensivamente porque las papas con contenidos elevados de azúcares reductores producen productos indeseables de color oscuro y acrilamida, sospechosa de cancerígena, durante el cocinado a alta temperatura. Las papas, en almacenamientos comerciales se enfrían lentamente, pero muchos estudios e investigaciones han usado papas enfriadas rápidamente. En este estudio se examinaron los niveles de enfriamiento y la variedad en el color de la hojuela, azucares y expresión de genes. Los contenidos de sacarosa y de azucares reductores fueron substancialmente más bajos en tubérculos enfriados lentamente que los enfriados rápidamente de “Snowden” y “MegaChip” por las primeras once semanas después de que empezó el enfriamiento a 3 °C. Se observaron diferencias en la expresión de genes para VInv, β-amylasa, SPS, AGPasa y GBSS entre tratamientos de enfriamiento y variedades. En general, los datos mostraron que el nivel de enfriamiento, el tiempo de almacenamiento y la variedad influenciaron múltiples aspectos del endulzamiento inducido por el frío.







Similar content being viewed by others
References
Amrein, T., S. Bachmann, A. Noti, M. Biederman, M. Barbosa, S. Biedermann-Brem, K. Grob, A. Keiser, P. Realini, F. Escher, and R. Amado. 2003. Potential of acrylamide formation, sugars, and free asparagine in potatoes: A comparison of cultivars and farming systems. Journal of Agricultural and Food Chemistry 51: 5556–5560.
Bagnaresi, P., A. Moschella, O. Beretta, F. Vitulli, P. Ranalli, and P. Perata. 2008. Heterologous microarray experiments allow the identification of the early events associated with potato tuber cold sweetening. BMC Genomics 9: 176.
Bethke, P.C. 2014. Postharvest storage and physiology. In The potato: Botany, production and uses, ed. M.J. Pavek and D.A. Navarre, 255–271. Wallingford: CABI.
Bethke, P.C., and A.J. Bussan. 2013. Acrylamide in processed potato products. American Journal of Potato Research 90: 403–424.
Bethke, P.C., R. Sabba, and A.J. Bussan. 2009. Tuber water and pressure potentials decrease and sucrose contents increase in response to moderate drought and heat stress. American Journal of Potato Research 86: 519–532.
Bhaskar, P.B., L. Wu, J.S. Busse, B.R. Whitty, A.J. Hamernik, S.H. Jansky, C.R. Buell, P.C. Bethke, and J. Jiang. 2010. Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiology 154: 939–948.
Biedermann-Brem, S., A. Noti, K. Grob, D. Imhof, D. Bazzocco, and A. Pfefferle. 2003. How much reducing sugar may potatoes contain to avoid excessive acrylamide formation during roasting and baking? European Food Research and Technology 217: 369–373.
Blenkinsop, R.W., L.J. Copp, R.Y. Yada, and A.G. Marangoni. 2002. Effect of chlorpropham (CIPC) on carbohydrate metabolism of potato tubers during storage. Food Research International 35: 651–655.
Brook, R.C., R.J. Fick, and T.D. Forbush. 1995. Potato storage design and management. American Journal of Potato Research 72: 463–480.
Brummell, D.A., R.K.Y. Chen, J.C. Harris, H. Zhang, C. Hamiaux, A.V. Kralicek, and M.J. McKenzie. 2011. Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. Journal of Experimental Botany 62: 3519–3534.
Burton, W.G. 1969. The sugar balance in some British potato varieties during storage. II. The effects of tuber age, previous storage temperature, and intermittent refrigeration upon low-temperature sweetening. European Potato Journal 12: 81–95.
Chen, X., F. Salamini, and C. Gebhardt. 2001. A potato molecular-function map for carbohydrate metabolism and transport. Theoretical and Applied Genetics 102: 284–295.
Chen, S., M.R. Hajirezaei, M.-I. Zanor, C. Hornyik, S. Debast, C. Lacomme, A.R. Fernie, U. Sonnewald, and F. Börnke. 2008. RNA interference-mediated repression of sucrose-phosphatase in transgenic potato tubers (Solanum tuberosum) strongly affects the hexose-to-sucrose ratio upon cold storage with only minor effects on total soluble carbohydrate accumulation. Plant, Cell & Environment 31: 165–176.
Copp, L.J., R.W. Blenkinsop, R.Y. Yada, and A.G. Marangoni. 2000. The relationship between respiration and chip color during long-term storage of potato tubers. American Journal of Potato Research 77: 279–287.
Deiting, U., R. Zrenner, and M. Stitt. 1998. Similar temperature requirement for sugar accumulation and for the induction of new forms of sucrose phosphate synthase and amylase in cold-stored potato tubers. Plant, Cell & Environment 21: 127–138.
Forbush, T., and R. Brook. 1993. Influence of airflow rate on chip potato storage management. American Journal of Potato Research 70: 869–883.
Galani Yamdeu, J.H., P.H. Gupta, A.K. Shah, N.J. Patel, and J.G. Talati. 2015. Profiling of StvacINV1, Bam1 and INH2α expressions in relation to acid invertase and β-amylase activities during development of cold-induced sweetening in Indian potato (Solanum tuberosum L.) tubers. American Journal of Potato Research 92: 603–608.
Groza, H.I., B.D. Bowen, A.J. Bussan, W.R. Stevenson, F. Navarro, D. Kichefski, S.J. Peloquin, J. Palta, and J. Jiang. 2007. Megachip – A new potato variety for chipping. American Journal of Potato Research 84: 343–350.
He, T., B. Song, J. Lin, X. Chen, Y. Ou, Y. Lin, H. Zhang, and C. Xie. 2012. A new isoform of thioredoxin h group in potato, SbTRXh1, regulates cold-induced sweetening of potato tubers by adjusting sucrose content. Plant Cell Reports 31: 1463–1471.
Hill, L.M., R. Reimholz, R. Schröder, T.H. Nielsen, and M. Stitt. 1996. The onset of sucrose accumulation in cold-stored potato tubers is caused by an increased rate of sucrose synthesis and coincides with low levels of hexose-phosphates, an activation of sucrose phosphate synthase and the appearance of a new form of amylase. Plant, Cell & Environment 19: 1223–1237.
Hogervorst, J.G.F., B.-J. Baars, L.J. Schouten, E.J.M. Konings, R.A. Goldbohm, and P.A. van den Brandt. 2010. The carcinogenicity of dietary acrylamide intake: A comparative discussion of epidemiological and experimental animal research. Critical Reviews in Toxicology 40: 485–512.
Holley, J. 2003. Storage cycle. In Guide to Commercial Potato Production in the Canadian Prairies. Published by Western Potato Council. www.gov.mb.ca/agriculture/crops/production/pubs/guide-to-commercial-potato-production.pdf. Accessed 10 February 2017.
Krause, K.-P., L. Hill, R. Reimholz, T. Hamborg-Nielsen, U. Sonnewald, and M. Stitt. 1998. Sucrose metabolism in cold-stored potato tubers with decreased expression of sucrose phosphate synthase. Plant, Cell & Environment 21: 285–299.
Maillard, L.-C. 1912. Action des acides amines sur les sucres; formation des mélanoïdines par voie méthodique. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 154: 66–68.
Matsuura-Endo, C., A. Kobayashi, T. Noda, S. Takigawa, H. Yamaguchi, and M. Mori. 2004. Changes in sugar content and activity of vacuolar acid invertase during low-temperature storage of potato tubers from six Japanese cultivars. Journal of Plant Research 117: 131–137.
Mottram, D.S., B.L. Wedzicha, and A.T. Dodson. 2002. Acrylamide is formed in the Maillard reaction. Nature 419: 448–449.
NASS-USDA. 2015. Potatoes 2014 Summary (September 2015). Washington, DC: United States Department of Agriculture, National Agricultural Statistics Service.
Nielsen, T.H., U. Deiting, and M. Stitt. 1997. A β-amylase in potato tubers is induced by storage at low temperature. Plant Physiology 113: 503–510.
Ou, Y., B. Song, X. Liu, Y. Lin, H. Zhang, M. Li, H. Fang, and J. Liu. 2013. Profiling of StvacINV1 expression in relation to acid invertase activity and sugar accumulation in potato cold-stored tubers. Potato Research 56: 157–165.
Peloquin, S.J., C. Thill, and A.D. Pavlista. 1994. Cultivars: Snowden. Nebraska Potato Eyes 6: 2–3.
Pressey, R. 1969. Role of invertase in the accumulation of sugars in cold-stored potatoes. American Potato Journal 46: 291–297.
Pressey, R. 1970. Changes in sucrose synthetase and sucrose phosphate synthase activities during storage of potatoes. American Potato Journal 47: 245–251.
Pritchard, M.K., and L.R. Adam. 1992. Preconditioning and storage of chemically immature russet Burbank and Shepody potatoes. American Potato Journal 69: 805–815.
Schreiber, L., A.C. Nader-Nieto, E.M. Schonhals, B. Walkemeier, and C. Gebhardt. 2014. SNPs in genes functional in starch-sugar interconversion associate with natural variation of tuber starch and sugar content of potato (Solanum tuberosum L.). G3 4: 1797–1811.
Shallenberger, R.S., O. Smith, and R.H. Treadway. 1959. Food color changes, role of the sugars in the browning reaction in potato chips. Journal of Agricultural and Food Chemistry 7: 274–277.
Sowokinos, J.R. 2001. Biochemical and molecular control of cold-induced sweetening in potatoes. American Journal of Potato Research 78: 221–236.
Sowokinos, J.R. and D.A. Preston. 1988. Maintenance of potato processing quality by chemical maturity monitoring (CMM). St. Paul, MN: University of Minnesota Agricultural Experiment Station. Bulletin 586–1988.
Stark, J.C. and S.L. Love (eds). 2003. Potato production systems. Moscow, ID: University of Idaho Agricultural Communications.
Stark, D.M., G.F. Barry, and G.M. Kishore. 1996. Improvement of food quality traits through enhancement of starch biosynthesis. Annals of the New York Academy of Sciences 792: 26–36.
Walkof, C., and B.B. Chubey. 1969. Relationship of chipping quality of potatoes to maturity and storage temperature. Canadian Journal of Plant Science 49: 453–458.
Walsh, J.R. 1995. Utilizing the stored crop. American Potato Journal 72: 481–492.
Wiberley-Bradford, A.E., J.S. Busse, and P.C. Bethke. 2016. Temperature-dependent regulation of sugar metabolism in wild-type and low-invertase transgenic chipping potatoes during and after cooling for low-temperature storage. Postharvest Biology and Technology 115: 60–71.
Wu, L., Bhaskar, P. B., Busse, J. S., Zhang, R., Bethke, P. C., & Jiang, J. (2011). Developing cold-chipping potato varieties by silencing the vacuolar invertase gene. Crop Science 51(3), 981–990 doi:10.2135/cropsci2010.08.0473.
Yang, J., J.R. Powers, T.D. Boylston, and K.M. Weller. 1999. Sugars and free amino acids in stored russet Burbank potatoes treated with CIPC and alternative sprout inhibitors. Journal of Food Science 64: 592–596.
Zhang, H., J. Hou, J. Liu, C. Xie, and B. Song. 2014. Amylase analysis in potato starch degradation during cold storage and sprouting. Potato Research 57: 47–58.
Zrenner, R., K. Schüler, and U. Sonnewald. 1996. Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198: 246–252.
Acknowledgements
The authors thank James Busse and Megan Evansen for assistance with sample collection and processing. Financial support for this project was provided by USDA-NIFA-SCRI Grant No. 2011-51181-30629 (Improved Breeding and Variety Evaluation Methods to Reduce Acrylamide Content and Increase Quality in Processed Potato Products) and by Potatoes USA. All experiments complied with the laws of the United States of America, where they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiberley-Bradford, A.E., Bethke, P.C. Rate of Cooling Alters Chip Color, Sugar Contents, and Gene Expression Profiles in Stored Potato Tubers. Am. J. Potato Res. 94, 534–543 (2017). https://doi.org/10.1007/s12230-017-9591-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-017-9591-3