Abstract
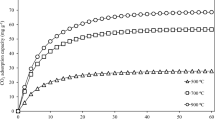
This paper was aimed to study the influence of modification of biochar on the performance of CO2 adsorption. Biochar, obtained from cotton stalk pyrolysis in a fixed bed reactor, was modified with ammonia and CO2. The physicochemical properties of biochars were characterized by the Fourier transform infrared spectroscopy and automatic adsorption equipment (Micromeritics, ASAP 2020, USA). CO2 adsorption of biochar was performed in thermogravimetric analyzer. The results showed that the surface area of char was increased significantly by CO2 modification, while N-contained compound on char surface was enriched obviously by NH3 modification. CO2 adsorption of biochar increased greatly with CO2 and NH3 modification. CO2 adsorption was mainly attributed to physical adsorption at 20 °C, and the adsorption quantity (maximum = 99 mg/g) was proportional to the micropore volume of the char. However, at 120 °C, molecular thermal motion increase, chemical adsorption start to play a dominated role, and the adsorption was directly proportional to the N content of this char.






Similar content being viewed by others
References
Brunner G (2009) Near critical and supercritical water, Part I. Hydrolytic and hydrothermal processes. J Supercrit Fluids 47(3):373–381
Drage TC, Arenillas A, Smith KM, Pevida C, Piippo S, Snape CE (2007) Preparation of carbon dioxide adsorbents from the chemical activation of urea–formaldehyde and melamine–formaldehyde resins. Fuel 86(1):22–31
Li W, Choi S, Drese JH, Hornbostel M, Krishnan G, Eisenberger PM, Jones CW (2010) Steam-stripping for regeneration of supported amine-based CO2 adsorbents. Chem Sus Chem 3(8):899–903
Plaza M, Pevida C, Arenillas A, Rubiera F, Pis J (2007) CO2 capture by adsorption with nitrogen enriched carbons. Fuel 86(14):2204–2212
Arenillas A, Smith K, Drage T, Snape C (2005) CO2 capture using some fly ash-derived carbon materials. Fuel 84(17):2204–2210
Liang Z, Marshall M, Chaffee AL (2009) CO2 adsorption-based separation by metal organic framework (Cu-BTC) versus zeolite (13X). Energy Fuel 23(5):2785–2789
Plaza M, Pevida C, Arias B, Casal M, Martín C, Fermoso J, Rubiera F, Pis J (2009) Different approaches for the development of low-cost CO2 adsorbents. J Environ Eng 135(6):426–432
Xu X, Song C, Andresen JM, Miller BG, Scaroni AW (2002) Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energ Fuels 16(6):1463–1469
Plaza M, Rubiera F, Pis J, Pevida C (2010) Ammoxidation of carbon materials for CO2 capture. Appl Surf Sci 256(22):6843–6849
Yang RT (1986) Gas separation by adsorption processes. Butterworth, Stoneham
Rodriguez-Reinoso F, López-González JD, Berenguer C (1984) Activated carbons from almond shells—II: characterization of the pore structure. Carbon 22(1):13–18
Rodriguez-Reinoso F, Molina-Sabio M (1992) Activated carbons from lignocellulosic materials by chemical and/or physical activation: an overview. Carbon 30(7):1111–1118
Liu WJ, Zeng FX, Jiang H, Zhang XS (2011) Preparation of high adsorption capacity bio-chars from waste biomass. Bioresour Technol 102(17):8247–8252
Swiatkowski A, Pakula M, Biniak S, Walczyk M (2004) Influence of the surface chemistry of modified activated carbon on its electrochemical behaviour in the presence of lead(II) ions. Carbon 42(15):3057–3069
Bagreev A, Bashkova S, Bandosz TJ (2002) Adsorption of SO2 on activated carbons: the effect of nitrogen functionality and pore sizes. Langmuir 18(4):1257–1264
Adib F, Bagreev A, Bandosz TJ (2000) Adsorption/oxidation of hydrogen sulfide on nitrogen-containing activated carbons. Langmuir 16(4):1980–1986
Maroto-Valer MM, Tang Z, Zhang Y (2005) CO2 capture by activated and impregnated anthracites. Fuel Process Technol 86(14):1487–1502
Mercedes Maroto-Valer M, Lu Z, Zhang Y, Tang Z (2008) Sorbents for CO2 capture from high carbon fly ashes. Waste Manag 28(11):2320–2328
Pevida C, Plaza M, Arias B, Fermoso J, Rubiera F, Pis J (2008) Surface modification of activated carbons for CO2 capture. Appl Surf Sci 254(22):7165–7172
Plaza M, Pevida C, Arias B, Fermoso J, Arenillas A, Rubiera F, Pis J (2008) Application of thermogravimetric analysis to the evaluation of aminated solid sorbents for CO2 capture. J Therm Anal Calorim 92(2):601–606
Przepiorski J, Skrodzewicz M, Morawski A (2004) High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Appl Surf Sci 225(1):235–242
Biniak S, Szymański G, Siedlewski J, Światkowski A (1997) The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 35(12):1799–1810
Bota KB, Abotsi GMK (1994) Ammonia: a reactive medium for catalysed coal gasification. Fuel 73(8):1354–1357
Boehm HP, Mair G, Stoehr T, De Rincón AR, Tereczki B (1984) Carbon as a catalyst in oxidation reactions and hydrogen halide elimination reactions. Fuel 63(8):1061–1063
Jansen R, Van Bekkum H (1994) Amination and ammoxidation of activated carbons. Carbon 32(8):1507–1516
Meldrum BJ, Rochester CH (1990) In situ infrared study of the surface oxidation of activated carbon in oxygen and carbon dioxide. J Chem Soc Faraday Trans 86(5):861–865
Vinke P, Van der Eijk M, Verbree M, Voskamp A, Van Bekkum H (1994) Modification of the surfaces of a gas activated carbon and a chemically activated carbon with nitric acid, hypochlorite, and ammonia. Carbon 32(4):675–686
Stöhr B, Boehm H, Schlögl R (1991) Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate. Carbon 29(6):707–720
Grant KA, Zhu Q, Thomas KM (1994) Nitrogen release in the gasification of carbons. Carbon 32(5):883–895
Jansen R, Van Bekkum H (1995) XPS of nitrogen-containing functional groups on activated carbon. Carbon 33(8):1021–1027
Chen Y, Wang X, Chen H, Yang H, Zhang S (2012) Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: influence of temperature. Bioresour Technol 107:411–418
Plaza M, Pevida C, Arias B, Fermoso J, Rubiera F, Pis J (2009) A comparison of two methods for producing CO2 capture adsorbents. Energy Procedia 1(1):1107–1113
Shafeeyan MS, Daud WMAW, Houshmand A, Arami-Niya A (2011) Ammonia modification of activated carbon to enhance carbon dioxide adsorption: effect of pre-oxidation. Appl Surf Sci 257(9):3936–3942
Moreno-Castilla C, Ferro-Garcia M, Joly J, Bautista-Toledo I, Carrasco-Marin F, Rivera-Utrilla J (1995) Activated carbon surface modifications by nitric acid, hydrogen peroxide, and ammonium peroxydisulfate treatments. Langmuir 11(11):4386–4392
Chen JP, Wu S (2004) Acid/base-treated activated carbons: characterization of functional groups and metal adsorptive properties. Langmuir 20(6):2233–2242
Pradhan BK, Sandle N (1999) Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 37(8):1323–1332
Plaza M, Pevida C, Martín C, Fermoso J, Pis J, Rubiera F (2010) Developing almond shell-derived activated carbons as CO2 adsorbents. Sep Purif Technol 71(1):102–106
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrol 89(2):143–151
Lazar G, Lazar I (2003) IR characterization of a-C:H:N films sputtered in Ar/CH4/N2 plasma. J Non-Cryst Solids 331(1):70–78
Mangun CL, Benak KR, Economy J, Foster KL (2001) Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 39(12):1809–1820
Hontoria-Lucas C, Lopez-Peinado A, López-González JD, Rojas-Cervantes M, Martin-Aranda R (1995) Study of oxygen-containing groups in a series of graphite oxides: physical and chemical characterization. Carbon 33(11):1585–1592
Kapoor A, Ritter J, Yang R (1989) On the Dubinin–Radushkevich equation for adsorption in microporous solids in the Henry’s law region. Langmuir 5(4):1118–1121
Acknowledgments
The author wishes to express her sincere thanks to the financial support from National Natural Science Foundation of China (51276075 and 51021065) and National Key Technology R&D Program in the 12th Five-Year Plan of China (2011BAD15B05-03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, Z., Shihong, Z., Haiping, Y. et al. Influence of NH3/CO2 Modification on the Characteristic of Biochar and the CO2 Capture. Bioenerg. Res. 6, 1147–1153 (2013). https://doi.org/10.1007/s12155-013-9304-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9304-9