Abstract
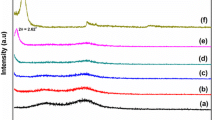
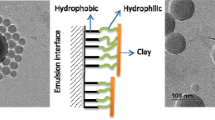
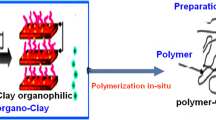
This study reports a steady-state fluorescence (SSF) technique for studying film formation from surfactant-free polystyrene (PS) latex and Na-montmorillonite (SNaM) composites. The composite films were prepared from pyrene (P)-labeled PS particles and SNaM clay at room temperature and annealed at elevated temperatures in 10-min intervals above glass transition temperature (t3) of polystyrene. During the annealing processes, the transparency of the film improved considerably. Scattered light (Is) and fluorescence intensity (Ip) from P were measured after each annealing step to monitor the stages of film formation. Evolution of transparency of composite films was monitored by using photon transmission intensity, Itr. Scanning electron microscopy (SEM) was used to detect the variation in physical structure of annealed composite films. Minimum film formation temperature, Tq, and healing temperatures, Th, were determined. Void closure and interdiffusion stages were modeled and related activation energies were determined. It was observed that both activation energies increased as the percent of SNaM was increased in composite films.
Similar content being viewed by others
References
Giannelis, E.P., Adv. Mater., 8, 29, 8 (1996).
Le Baron, P.C., Wang, Z., and Pinnavaia, T.J., Appl. Clay. Sci., 15, 11 (1999).
Alexandre, M. and Dubais, P., Mater. Sci. Eng., 28, 1 (2000).
Collister, J., in: Polymer Nanocomposites, Synthesis, Characterization and Modeling, Vaia, R.A. and Krishnamoorti, R., (Eds.), Oxford University Press, London, Chapter 2, 2002.
Kawasumi, M., Kohzaki, M., Kojima, Y., Okada, A., and Kamiyouto, O., U.S. Patent 4,810,734, 1989.
Usuki, A., Kojma, Y., Kawasumi, M., Okada, A., Fukuskima, Y., Kuvauchi, T., and Kamigato, O., J. Mater. Res., 8, 1179 (1993).
Sperry, P.R., Synder, B.S., O’Dowd, M.L., and Lesko, P.M., Langmuir, 10, 2619 (1994).
Mazur, S., “Coalescence of Polymer Particles,” Polymer Powder Processing, Rosenweig, N. (Ed.), Wiley and Sons, New York, 1995.
Mackenzie, J.K. and Shutleworth, R., Proc. Phys. Soc., London, 62, 838 (1946).
Vanderhoff, J.W., Br. Polym. J., 2, 161 (1970).
Kanig, G. and Neff, H., Colloid Polym. Sci., 256, 1052 (1975).
Wang, Y., Kats, A., Juhue, D., Winnik, M.A., Shivers, R.R., and Dinsdale, C.J., Langmuit, 8, 1435 (1992).
Roulstone, B.J., Wilkinson, M.C., Hearn, J., and Wilson, A.J., Polym. Int., 24, 87 (1991).
Kim, K.D., Sperling, L.H., and Klein, A., Macromolecules, 26, 4624 (1993).
Pekcan, Ö., Winnik, M.A., and Croucher, M.D., Macromolecules, 23, 2673 (1990).
Wang, Y., Zaho, C.L., and Winnik, M.A., J. Chem. Phys., 95, 2143 (1991).
Wang, Y. and Winnik, M.A., Macromolecules, 26, 3147 (1993).
Pekcan, Ö. and Canpolat, M., J. Appl. Polym. Sci., 59, 277 (1996).
Pekcan, Ö., Canpolat, M., and Göçmen, A., Polymer, 34, 3319 (1993).
Canpolat, M. and Pekcan, Ö., J. Polym. Sci. B, Polym. Phys., 34, 691 (1996).
Ardad, E., Bulmus, V., Piskin, E., and Pekcan, Ö., J. Colloid Interface Sci., 213, 160 (1999).
Pekcan, Ö. and Arda, E., Colloids and Surf. A, 153, 537 (1999).
Arranda, P. and Ruiz-Hitzky, E., Chem. Water, 4, 1395 (1992).
Wu, J. and Lerner, M.M., Chem. Mater., 5, 835 (1993).
Vaia, R.A., Vasudevan, S., Krawiec, W., Scalon, L.G., and Giannelis, E.P., Adv. Mater., 7, 154 (1995).
Kawasumi, M., Hasegawa, N., Kato, M., Ususki, A., and Okada, A., Macromolecules, 30, 6333 (1997).
Wang, Y., Zhang, Q., and Fu, Q., Macromol. Rapid Commun., 24, 231 (2003).
alemdar, A., “The Effect of Organic and Inorganic Additives on the Rheological and Colloidal Properties of Bentonite and Montmorillonite Dispersions,” Ph.D. Thesis, (2001).
Tributh, H. and Lagaly, G., “Aufbereitung und Identifizierung von Boden-und Lagerstattenttonen GIT Fachzeitschrift für das,” Laboratorium, 30, 524–529 771–776, (1986).
Stul, M.S. and Van Leemput, L., Clay Miner., 17, 209 (1982).
Lagaly, G., “Layer Charge Determination by alkylammonium Ions,” CMS Workshop Lectures, Vol. 6, Mermut, A.R. (Ed.), 1994.
Olphen, V.H., An Introduction to Clay Colloid Chemistry, 2nd Ed. Interscience Publishers, New York, 1977.
Grim, R.E., Applied Clay Minerology, McGraw-Jill book Company Inc., New York, 1962.
Ece, Ö.I., Alemdar, A., Güngör, N., and Hayashi, S., J. Appl. Polym. Sci., 86, 341 (2002).
Keddie, J.L., Meredith, P., Jones, R.A.L., and Donald, A.M., Film Formation in Waterborne Coatings, Provder, T., Winnik, M.A. and Urban, M.W., (Eds.), ACS Symp. Ser., 648. pp. 332–348. Amer. Chem. Soc., 1996.
McKenna, G.B., In Comprehensive Polymer Science, Vol. 2, Booth, C. and Price, C. (Eds.), Pergamon Press, Oxford UK, 1989.
Vogel, H., Phys. Z. 22, 645 (1925).
Fulcher, G.S., J. Phys. USSR, 9, 385 (1945).
Frenkel, J., J. Phys. USSR, 9, 385 (1945).
Voyutskii, S. Colloid Chemistry, MIR Publisher, Moscow, 1963.
Prager, S. and Tirrell, M., J. Chem. Phys., 75, 5194 (1981).
Wool, R.P., Yuan, B.L., and McGarel, O.J., J. Polym. Eng. Sci., 29, 1340 (1989).
de Gennes, P.G., J. Chem. Phys., 76, 3322 (1982).
Kim, Y.H. and Wool, R.P., Macromolecules, 16, 1115 (1983).
Wool, R.P. and O’Connor, K.M., J. Appl. Phys., 52, 5953 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ugur, S., Alemdar, A. & Pekcan, Ö. Films formed from polystyrene latex/clay composites: A fluorescence study. J Coat. Technol. Res. 2, 565–575 (2005). https://doi.org/10.1007/s11998-005-0016-8
Issue Date:
DOI: https://doi.org/10.1007/s11998-005-0016-8