Abstract
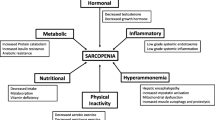
Sarcopenia, a loss of muscle mass, is being increasingly recognized to have a deleterious effect on outcomes in patients with chronic liver disease. Factors related to diet and the inflammatory nature of chronic liver disease contribute to the occurrence of sarcopenia in these patients. Sarcopenia adversely influences quality of life, performance, morbidity, success of transplantation, and even mortality. Specific deficiencies in macronutrients (protein, polyunsaturated fatty acids) and micronutrients (vitamins C, D, and E, carotenoids, and selenium) have been linked to sarcopenia. Lessons learned from nutritional therapy in geriatric patient populations may provide strategies to manage sarcopenia in patients with liver disease. Combining diet modification and nutrient supplementation with an organized program of exercise may help ameliorate or even reverse the effects of sarcopenia on an already complex disease process.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance,•• Of major importance
Wolfe RA, McCullough KP, Leichtman AB. Predictability of survival models for waiting list and transplant patients: calculating LYFT. Am J Transplant. 2009;9(7):1523–7.
Schaubel DE et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(4 Pt 2):970–81.
Sharma P et al. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135(5):1575–81.
Montano-Loza AJ et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20(6):640–8. Recent work that demonstrates the impact of sarcopenia on survival at the time of transplant. Sarcopenia is emerging as a biomarker for malnutrition.
Cruz-Jentoft AJ et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129–33.
Jancova-Vseteckova J et al. Social patterning in grip strength, chair rise, and walk speed in an aging population: the Czech HAPIEE Study. J Aging Phys Act. 2015;23(2):264–71.
Syddall H et al. Social inequalities in grip strength, physical function, and falls among community dwelling older men and women: findings from the Hertfordshire Cohort Study. J Aging Health. 2009;21(6):913–39.
Nieuwenhuizen WF et al. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr. 2010;29(2):160–9.
Murphy C. The chemical senses and nutrition in older adults. J Nutr Elder. 2008;27(3–4):247–65.
Kachaamy T, Bajaj JS, Heuman DM. Muscle and mortality in cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):100–2. A review paper which nicely summarizes the impact of muscle fitness on mortality in cirrhosis.
Mendenhall CL et al. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. 1984;76(2):211–22.
Peng S et al. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85(5):1257–66.
Aberg F, Isoniemi H, Hockerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100(1):14–21.
Duffy JP et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252(4):652–61.
Dasarathy S. Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci. 2013;58(11):3103–11. This study explores the notion that sarcopenia is an entity independent of liver disease, and that intervention may have a reversible impact on survival after transplant.
Kaiser M, Bandinelli S, Lunenfeld B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Biomed. 2010;81 Suppl 1:37–45.
Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–19S.
Volpi E et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8.
Smith K et al. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275(1 Pt 1):E73–8.
Volpi E et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277(3 Pt 1):E513–20.
Marchesini G et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124(7):1792–801.
Muto Y et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3(7):705–13.
Yoshida T et al. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol Jpn. 1989;24(6):692–8.
Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135(6 Suppl):1539S–46S.
Skeie B et al. Branch-chain amino acids: their metabolism and clinical utility. Crit Care Med. 1990;18(5):549–71.
Tsien C et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61(6):2018–29. Amino acid supplementation altering muscle anabolism in patient with alcoholic liver disease. Highlights work being done in the area of nutritional therapy in liver disease.
Annweiler C et al. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13(10):893–8.
Geusens P et al. Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res. 1997;12(12):2082–8.
Wilhelm-Leen ER et al. Vitamin D deficiency and frailty in older Americans. J Intern Med. 2010;268(2):171–80.
Bischoff-Ferrari HA et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692.
Kim JS, Wilson JM, Lee SR. Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutr Biochem. 2010;21(1):1–13.
Semba RD et al. Oxidative stress and severe walking disability among older women. Am J Med. 2007;120(12):1084–9.
Lauretani F et al. Carotenoids as protection against disability in older persons. Rejuvenation Res. 2008;11(3):557–63.
Chae CH, Shin CH, Kim HT. The combination of alpha-lipoic acid supplementation and aerobic exercise inhibits lipid peroxidation in rat skeletal muscles. Nutr Res. 2008;28(6):399–405.
Jakeman P, Maxwell S. Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur J Appl Physiol Occup Physiol. 1993;67(5):426–30.
Nakhostin-Roohi B et al. Effect of vitamin C supplementation on lipid peroxidation, muscle damage and inflammation after 30-min exercise at 75% VO2max. J Sports Med Phys Fitness. 2008;48(2):217–24.
Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. 2011;41(12):1043–69.
Marshall RJ et al. Supplemental vitamin C appears to slow racing greyhounds. J Nutr. 2002;132(6 Suppl 2):1616S–21S.
Owen OE et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981;68(1):240–52.
Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27(3):430–41.
Morihara D et al. Late-evening snack with branched-chain amino acids improves liver function after radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2012;42(7):658–67.
Sorrentino P et al. Preservation of nutritional-status in patients with refractory ascites due to hepatic cirrhosis who are undergoing repeated paracentesis. J Gastroenterol Hepatol. 2012;27(4):813–22.
Gheorghe L et al. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol. 2005;14(3):231–8.
Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015;31(4):549–55.
Murphy RA et al. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer. 2011;117(8):1775–82.
Rodacki CL et al. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr. 2012;95(2):428–36.
Smith GI et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402–12.
Capel F et al. DHA at nutritional doses restores insulin sensitivity in skeletal muscle by preventing lipotoxicity and inflammation. J Nutr Biochem. 2015;26(9):949–59.
Dewey A et al. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 2007;1, CD004597.
Cruz Jr RJ et al. Objective radiologic assessment of body composition in patients with end-stage liver disease: going beyond the BMI. Transplantation. 2013;95(4):617–22.
Dharancy S et al. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86(8):1077–83.
DiMartini A et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19(11):1172–80.
Durand F et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60(6):1151–7.
Englesbe MJ et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–8.
Epstein SK et al. Aerobic capacity is associated with 100-day outcome after hepatic transplantation. Liver Transpl. 2004;10(3):418–24.
Lai JC et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14(8):1870–9.
Montano-Loza AJ et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166–73. 173 e1.
Ow MM et al. Impaired functional capacity in potential liver transplant candidates predicts short-term mortality before transplantation. Liver Transpl. 2014;20(9):1081–8.
Tandon P et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18(10):1209–16.
Hayashi F et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol Res. 2013;43(12):1264–75. Current work that demonstrates the value of exercise-based intervention on reversal of sarcopenia.
Montero-Fernandez N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. 2013;49(1):131–43.
Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6(1):87–93.
Campillo B et al. Submaximal oxygen consumption in liver cirrhosis. Evidence of severe functional aerobic impairment. J Hepatol. 1990;10(2):163–7.
Ritland S et al. Improvement of physical capacity after long-term training in patients with chronic active hepatitis. Scand J Gastroenterol. 1983;18(8):1083–7.
Shimomura Y et al. Branched-chain amino acid catabolism in exercise and liver disease. J Nutr. 2006;136(1 Suppl):250S–3S.
Shimomura Y et al. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr. 2004;134(6 Suppl):1583S–7S.
Bernal W et al. Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transpl. 2014;20(1):54–62. Interesting look at the effect cardiopulmonary rehabilitation has on patients with cirrhosis whether or not they actually receive a liver transplant.
Lemyze M, Dharancy S, Wallaert B. Response to exercise in patients with liver cirrhosis: implications for liver transplantation. Dig Liver Dis. 2013;45(5):362–6.
Roman E et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59(8):1966–75.
Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52(9):2191–7.
Hickman IJ et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53(3):413–9.
Konishi I et al. Aerobic exercise improves insulin resistance and decreases body fat and serum levels of leptin in patients with hepatitis C virus. Hepatol Res. 2011;41(10):928–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the topical collection on Nutrition and Obesity
An erratum to this article can be found at http://dx.doi.org/10.1007/s11894-016-0533-x.
Rights and permissions
About this article
Cite this article
Kappus, M.R., Mendoza, M.S., Nguyen, D. et al. Sarcopenia in Patients with Chronic Liver Disease: Can It Be Altered by Diet and Exercise?. Curr Gastroenterol Rep 18, 43 (2016). https://doi.org/10.1007/s11894-016-0516-y
Published:
DOI: https://doi.org/10.1007/s11894-016-0516-y