Abstract
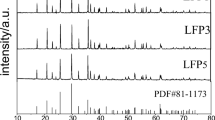
LiFePO4 was prepared by heating the pre-decomposed precursor mixtures sealed in vacuum quartz-tube. Three kinds of cooling modes including nature cooling, air quenching, and water quenching were applied to comparing the effects of cooling modes on the microstructure and electrochemical characteristics of the material. The results indicate that the water quenching mode can control overgrowth of the grain size of final product and improve its electrochemical performance compared with nature cooling mode and air quenching mode. The sample synthesized by using water quenching mode is of the highest reversible discharge specific capacity and the best cyclic electrochemical performance, demonstrating the first discharge capacity of 138.1 mA·h/g at 0.1C rate and the total loss of capacity of 3.11% after 20 cycles.
Similar content being viewed by others
References
NISHI Y. Lithium ion batteries: Past 10 years and the future[J]. J Power Sources, 2001, 100(1/2): 101–106.
PADHI A K, NAJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. J Electrochem Soc, 1997, 144(4): 1188–1194.
BAUER E M, BELLITTO C, PASQUALI M, et al. Versatile synthesis of carbon-rich LiFePO4 enhancing its electrochemical properties[J]. Electrochem Solid-State Lett, 2004, 7(4): A85–A90.
ZAGHIB K, STRIEBEL K, GUERFI A, et al. LiFePO4/polymer/natural graphite: Low cost Li-ion batteries[J]. Electrochimica Acta, 2004, 50(2/3): 263–270.
HUANG H, YIN S C, NAZAR L F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates[J]. Electrochem Solid-State Lett, 2001, 4(10): A170–A172.
GUERFI A, KANEKO M, PETITCLERC M, et al. LiFePO4 water-soluble binder electrode for Li-ion batteries[J]. J Power Sources, 2007, 163(2): 1047–1052.
HU Guo-rong, GAO Xu-guang, PENG Zhong-dong, et al. Synthesis of LiFePO4/C composite electrode with enhanced electrochemical performance[J]. Trans Nonferrous Met Soc China, 2005, 15(4): 795–798.
MYUNG S T, KOMABA S, HIROSAKI N, et al. Emulsion drying synthesis of olivine LiFePO4/C composite and its electrochemical properties as lithium intercalation material[J]. Electrochimica Acta, 2004, 49(24): 4213–4222.
HO C S, WON C, HO J. Electrochemical properties of carbon-coated LiFePO4 cathode using graphite, carbon black, and acetylene black[J]. Electrochimica Acta, 2006, 52(4): 1472–1476.
YANG Shou-feng, ZAVALIJ P Y, WHITTINGGHAM M S. Hydrothermal synthesis of lithium iron phosphate cathodes[J]. Electrochemistry Commun, 2001, 3(9): 505–508.
PARK K S, KANG K T, LEE S B, et al. Synthesis of LiFePO4 with fine particle by co-precipitation method[J]. Materials Research Bulletin, 2004, 39(12): 1803–1810.
YAMADA A, CHUNG S C, HINOKUMA K. Optimized LiFePO4 for lithium battery cathodes[J]. J Electrochem Soc, 2001, 148(3): A224–A229.
CHUNG S Y, BLOCKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes[J]. Nature Mater, 2002, 2: 123–128.
HU Guo-rong, GAO Xu-guang, PENG Zhong-dong, et al. Influence of Ti ion doping on electrochemical properties of LiFePO4/C cathode material for lithium-ion batteries[J]. Trans Nonferrous Met Soc China, 2007, 17(2): 296–300.
HONG Jian, WANG Chun-sheng, KASAVAJJULA U. Kinetic behavior of LiFeMgPO4 cathode material for Li-ion batteries[J]. J Power Sources, 2006, 162(2): 1289–1296
WANG De-yu, LI Hong, SHI Si-qi, et al. Improving the rate performance of LiFePO4 by Fe-site doping[J]. Electrochimica Acta, 2005, 50(14): 2955–2958
RAVET N, ABOUIMRANE A, ARMAND M, et al. On the electronic conductivity of phospho-olivines as lithium storage electrodes[J]. Nature Mater, 2003, 2: 702–720.
SUBRAMANYA H P, ELLIS B, COOMBS N, et al. Nano-network electronic conduction in iron and nickel olivine phosphates[J]. Nature Mater, 2004, 3: 147–152.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(50604018) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Hu, Gr., Gao, Xg., Peng, Zd. et al. Effect of cooling modes on microstructure and electrochemical performance of LiFePO4 . J Cent. South Univ. Technol. 14, 647–650 (2007). https://doi.org/10.1007/s11771-007-0124-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-007-0124-y