Abstract
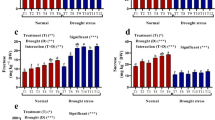
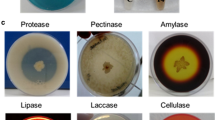
Plant growth promoting bacterial (PGPB) strains Pseudomonas fluorescens Pf1 and endophytic Bacillus subtilis EPB5, EPB22, EPB 31 were tested for their capacity to induce water stress related proteins and enzymes in green gram (Vigna radiata) plants. Among the different bacteria used, P. fluorescens Pf1 increased the vigour index, fresh weight and dry weight of green gram seedlings in vitro. Quantitative and qualitative analyses of stress-related enzymes indicated the greater activity of catalase and peroxidase in green gram plants bacterized with P. fluorescens Pf1 against water stress when compared to untreated plants. The greater accumulation of proline was recorded in Pf1 treated plants compared to untreated plants. The pot culture study revealed the greater resistance to water stress by green gram plants treated with P. fluorescens Pf1 compared to untreated plants. The greater activity of stress-related enzymes in green gram plants mediated by PGPB could pave the way for developing drought management strategies.





Similar content being viewed by others
References
Baki AAA, Anderson JD (1973) Vigour determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Barber JM (1980) Catalase and peroxidase in primary leaves during development and senescence. Z Pflanzenphysiol 97:135–144
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bowler C, van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Gaff DF (1997) Mechanisms of desiccation tolerance in resurrection vascular plants. In: Basra AS, Bastra RK (eds) Mechanisms of environmental stress resistance in plants. Harwood Academic Publishers, London, pp 43–58
Glick BR, Karaturovic DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth-promoting pseudomonads. Can J Microbiol 41:533–536
Gomez KA, Gomez AA (1984) Statistical procedure for agricultural research. Wiley, New York
Hammerschmidt R, Nuckles EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82
Hanson AD, Nelsen CE, Pedersen AR, Everson EH (1979) Capacity for proline accumulation during water stress in barley and its implications for breeding for drought resistance. Crop Sci 19:489–493
Indian Meteorological Department (2009) Present status of drought. Drought Bull 1:3–26
ISTA (1993) Proceedings of the International Seed Testing Association, international rules for seed testing. Seed Sci Technol 21:25–30
Kohler J, Hernàndez JA, Caravaca F, Roldàn A (2008) Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Fun Plant Biol 35:141–151
Mayak S, Tirosh T, Glick BR (2004) Plant growth promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci 166:525–530
Nandakumar R, Babu S, Viswanathan R, Raguchander T, Samiyappan R (2001) Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol Biochem 33:603–612
Quartacci MF, Pinzino C, Sgherri CLM, Dalla Vecchia F, Navari-Izzo F (2000) Growth in excess copper induces changes in the lipid composition and fluidity of PSII-enriched membranes in wheat. Physiol Plant 108:87–93
Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R (2001) Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pest and diseases. Crop Protect 20:1–11
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol 102:1283–1292
Saravanakumar D, Muthumeena B, Lavanya N, Suresh S, Rajendran L, Raguchander T, Samiyappan R (2007a) Pseudomonas induced defense molecules in rice against leaffolder (Cnephalocrocis medinalis) pest. Pest Manag Sci 63:714–721
Saravanakumar D, Vijayakumar C, Kumar N, Samiyappan R (2007b) PGPR induced defense responses in tea plants against blister blight disease. Crop Protect 26:556–565
Saravanakumar D, Lavanya N, Muthumeena K, Raguchander T, Samiyappan R (2009) Fluorescent pseudomonad mixtures mediate disease resistance in rice plants against sheath rot (Sarocladium oryzae) disease. Biocontrol 54:273–286
Scandalios JG (1994) Regulation and properties of plant catalases. In: Foyer CH, Mullineaux PM (eds) Causes of photo-oxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 275–316
Schellenbaum L, Mùller J, Boller T, Wienken A, Schùepp H (1998) Effects of drought on non-mycorrhizal and mycorrhizal maize: changes in the pools of non-structural carbohydrates, in the activities of invertase and trehalose, and in the pools of amino acids and imino acids. New Phytol 138:59–66
Sgherri CLM, Maffei M, Navari-Izzo F (2000) Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J Plant Physiol 157:273–279
Sindhu JS, Ravi S, Minocha JL (1984) Peroxidases isozyme patterns in primary trisomics of pearl millet. Theor Appl Genet 68:179–182
Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JD (1995) Molecular genetics of plant disease resistance. Science 268:661–667
Thompson DC (1996) Evaluation of bacterial antagonist for reduction of summer patch symptoms in Kentucky blue grass. Plant Dis 80:856–862
Vivekananthan R, Ravi M, Ramanathan A, Samiyappan R (2004) Lytic enzymes induced by Pseudomonas fluorescens and other biocontrol organisms mediate defence against the anthracnose pathogen in mango. World J Microbiol Biotechnol 20:235–244
Wang C, Knill E, Glick BR, De’fago G (2000) Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can J Microbiol 46:898–907
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozyme. Anal Biochem 44:301–305
Yang J, Kloepper JA, Ryu C (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Barna.
Rights and permissions
About this article
Cite this article
Saravanakumar, D., Kavino, M., Raguchander, T. et al. Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol Plant 33, 203–209 (2011). https://doi.org/10.1007/s11738-010-0539-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0539-1