Abstract
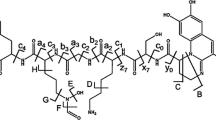
The fac-[Re(CO)3(4,4′-dimethyl-2,2′-bpy)L]PF6 (C2) complex have been recently reported as a useful fluorophore for walled cells (yeasts and bacteria) without the need of antibodies. In the present work, we report the structural parameters of the C2 complex, where L is an ancillary ligand E-2-((3-amino-pyridin-4-ylimino)-methyl)-4,6-di-tert-butylphenol, which presents an intramolecular hydrogen bond (IHB). The C2 crystals were obtained by slow evaporation of a dichloromethane solution, yielding yellow blocks. The crystal structure solution of the complex C2 showed a monoclinic crystal system and discrete organometallic cations and PF6 − as the counter ion, with partially occupation of solvent molecules (CH2Cl2). The complex C2 having a fac-geometry of the three carbonyl ligands, possesses the following bond distances Re–C(CO): Re1–C24, 1.87(8) Å; Re1–C25, 1.58(12) Å and Re1–C26, 1.90(8) Å. The distorted octahedral geometry observed in the C2 structure is due to the C(CO)–Re1–N1(imine) angle for the three carbonyls that are significantly different. The Re–N1 bond distance of 2.16(4) Å corresponds to the nitrogen coordination of the pyridine fragment of the ancillary ligand L, completing the octahedral geometry. Here we complement the C2 descriptions due to the considerable biological interest of its use as d6 metal fluorophore in walled cells (i.e., yeast and bacteria). DFT calculations were performed including scalar and spin–orbit (SO) relativistic effects with agree often reasonably well with experimental X-ray data. Through frequency calculations we estimated the strength of the intramolecular hydrogen bond (with a OH···N distance of 2.621 Å) accounting for near 40 kcal/mol, indicating that is a strong hydrogen bond which contributes to the molecular stability. In addition, we observed the L electron-withdrawing effect on the rhenium core. The agreement between the observed and computed bond distances and angles brings confidence on the choice of the computed models and level of theory. These kind of Rhenium (I) complexes designed to develop novel fluorophores suitable for biological applications.






Similar content being viewed by others
References
Agarwal J, Fujita E, Schaefer HF, Muckerman JT (2012) Mechanisms for CO production from CO2 using reduced rhenium tricarbonyl catalysts. J Am Chem Soc 134:5180–5186. doi:10.1021/ja2105834
Alvarado-Soto L, Ramírez-Tagle R, Arratia-Pérez R (2008) Spin–orbit effects on the aromaticity of the Re3Cl9 and Re3Br 9 clusters. Chem Phys Lett 467:94–96. doi:10.1016/j.cplett.2008.11.020
Amoroso AJ, Arthur RJ, Coogan MP, Court JB, Fernandez-Moreira V, Hayes AJ, Lloyd D, Millet C, Pope SJA (2008) 3-Chloromethylpyridyl bipyridine fac-tricarbonyl rhenium: a thiol-reactive luminophore for fluorescence microscopy accumulates in mitochondria. New J Chem 32:1097–1102. doi:10.1039/B802215A
(2007) Amsterdam Density Functional (ADF) Code. Vrije Universiteit, Amsterdam
Argazzi R, Bignozzi CA, Heimer TA, Meyer GJ (1997) Remote interfacial electron transfer from supramolecular sensitizers. Inorg Chem 36:2–3. doi:10.1021/ic960717g
Balakrishnan G, Rajendran T, Murugan KS, Kumar MS, Sivasubramanian VK, Ganesan M, Mahesh A, Thirunalasundari T, Rajagopal S (2015) Interaction of rhenium(I) complex carrying long alkyl chain with Calf Thymus DNA: cytotoxic and cell imaging studies. Inorg Chim Acta 434:51–59. doi:10.1016/j.ica.2015.04.036
Barrot M, Fabrias G, Camps F, Molins E, Roig A, Maniukiewlcz W (1998) 1,5,8,12-Tetrathiaspiro[6.6]tridecane. Act Cryst C54:533–534. doi:10.1107/S0108270197015138
Bera MK, Chakraborty C, Malik S (2016) Salen-based enantiomeric polymers for enantioselective recognition. New J Chem 40:8074–8080. doi:10.1039/C6NJ00844E
Bertrand HC, Clede S, Guillot R, Lambert F, Policar C (2014) Luminescence modulations of rhenium tricarbonyl complexes induced by structural variations. Inorg Chem 53:6204–6223. doi:10.1021/ic5007007
Bhuvaneswari J, Mareeswaran PM, Shanmugasundaram S, Rajagopal S (2011) Protein binding studies of luminescent rhenium(I) diimine complexes. Inorg Chim Acta 375:205–212. doi:10.1016/j.ica.2011.05.009
Bingol B, Durrell AC, Keller GE, Palmer JH, Grubbs RH, Gray HB (2013) Electron transfer triggered by optical excitation of phenothiazine-tris(meta-phenylene-ethynylene)-(tricarbonyl)(bpy)(py)rhenium(I). J Phys Chem B 117:4177–4182. doi:10.1021/jp3010053
Black DR, Hightower SE (2012) Preparation and characterization of rhenium(I) dicarbonyl complexes based on the meridionally coordinated terpyridine ligand. Inorg Chem Commun 24:16–19. doi:10.1016/j.inoche.2012.07.034
Carreño A, Preite M, Manriquez JM, Vega A, Chavez I (2010) Phenyl 3,5-di-tert-butyl-2-hydroxybenzoate. Act Cryst E66:o3290. doi:10.1107/S1600536810044028
Carreño A, Ladeira S, Castel A, Vega A, Chavez I (2012) (E)-2-{[(2-Aminopyridin-3-yl)imino]methyl}-4,6-di-tert-butylphenol. Act Cryst E66:o2507–o2508. doi:10.1107/S1600536812032060
Carreño A, Vega A, Zarate X, Schott E, Gacitua M, Valenzuela N, Preite M, Manriquez JM, Chavez I (2014) Synthesis, characterization and computational studies of (E)-2-{[(2-aminopyridin-3-yl)imino]-methyl}-4,6-di-tert-butylphenol, Quim. Nova 37:584–588. doi:10.5935/0100-4042.20140098
Carreño A, Gacitúa M, Páez-Hernández D, Polanco R, Preite M, Fuentes JA, Mora GC, Chávez I, Arratia-Pérez R (2015a) Spectral, theoretical characterization and antifungal properties of two phenol derivative Schiff bases with an intramolecular hydrogen bond. New J Chem 39:7822–7831. doi:10.1039/C5NJ01469G
Carreño A, Gacitua M, Schott E, Zarate X, Manriquez JM, Preite M, Ladeira S, Castel A, Pizarro N, Vega A, Chavez I, Arratia-Perez R (2015b) Experimental and theoretical studies of the ancillary ligand (E)-2-((3-amino-pyridin-4-ylimino)-methyl)-4,6-di-tert-butylphenol in the rhenium(I) core. New J Chem 39:5725–5734. doi:10.1039/C5NJ00772K
Carreño A, Gacitúa M, Fuentes JA, Páez-Hernández D, Peñaloza JP, Otero C, Preite M, Molins E, Swords WB, Meyer GJ, Manríquez JM, Polanco R, Chávez I, Arratia-Pérez R (2016a) Fluorescence probes for prokaryotic and eukaryotic cells using Re(CO) +3 complexes with an electron withdrawing ancillary ligand. New J Chem 40:7687–7700. doi:10.1039/C6NJ00905K
Carreño A, Gacitúa M, Fuentes JA, Páez-Hernández D, Araneda C, Chávez I, Soto-Arriaza M, Manriquez JM, Polanco R, Mora GC, Otero C, Swords WB, Arratia-Pérez R (2016b) Theoretical and experimental characterization of a novel pyridine benzimidazole: suitability for fluorescence staining in cells and antimicrobial properties. New J Chem 40:2362–2375. doi:10.1039/c5nj02772a
Carreño A, Schott E, Zarate X, Manriquez JM, Vega JC, Mardones M, Cowley AH, Chavez I, Hinestroza JP, Arratia-Perez R (2016c) DFT studies on coordination models for adsorption essays of Cu(II) and Ni(II) solutions in modified silica gel with iminodiacetic groups. Chem Pap. doi:10.1007/s11696-016-0022-6
Carreño A, Aros A, Otero C, Polanco R, Gacitua M, Arratia-perez R, Fuentes J (2017) Substituted bidentate and ancillary ligands modulate the bioimaging properties of the classical Re(I) tricarbonyl core with yeasts and bacteria. New J Chem 41:2140–2147. doi:10.1039/c6nj03792e
Carrington SJ, Chakraborty I, Bernard JML, Mascharak PK (2016) A theranostic two-tone luminescent photoCORM derived from Re(I) and (2-pyridyl)-benzothiazole: trackable CO delivery to Malignant cells. Inorg Chem 55:7852–7858. doi:10.1021/acs.inorgchem.6b00511
Cattaneo M, Vergara MM, García Posse ME, Fagalde F, Parella T, Katz NE (2014) Spectroscopic, electrochemical and computational studies of rhenium(I) and ruthenium(II) complexes incorporating the novel tetradentate ligand 1,4-bis(4-(4′-methyl)-2,2′-bipyridyl)-2,3-diaza-1,3-butadiene (BBDB) and its derivatives. Polyhedron 70:20–28. doi:10.1016/j.poly.2013.12.018
Chan CY, Pellegrini PA, Greguric I, Barnard PJ (2014) Rhenium and technetium tricarbonyl complexes of N-heterocyclic carbene ligands. Inorg Chem 53:10862–10873. doi:10.1021/ic500917s
Coogan MP, Platts JA (2016) Blue rhenium tricarbonyl DPPZ complexes—low energy charge-transfer absorption at tissue-penetrating wavelengths. Chem Commun 52:12498–12501. doi:10.1039/C6CC07125B
Cote C, Kirss RU (2010) Rhenium tricarbonyl complexes of 1-benzyl-4-(5-bipyridyl)-1H-1,2,3-triazoles. Inorg Chim Acta 363:2520–2525. doi:10.1016/j.ica.2010.04.019
Czerwieniec R, Kapturkiewicz A, Lipkowski J, Nowacki J (2005) Re(I)(tricarbonyl)+ complexes with the 2-(2-pyridyl)-N-methyl-benzimidazole, 2-(2-pyridyl)benzoxazole and 2-(2-pyridyl)benzothiazole ligands—syntheses, structures, electrochemical and spectroscopic studies. Inorg Chim Acta 358:2701–2710. doi:10.1016/j.ica.2005.03.013
Dashnau JL, Sharp KA, Vanderkooi JM (2005) Carbohydrate intramolecular hydrogen bonding cooperativity and its effect on water structure. J Phys Chem B 109:24152–24152. doi:10.1021/jp0543072
Emamian S, Tayyari SF (2013) Theoretical study of intramolecular hydrogen bonding in the halo derivatives of 1-amino-3-imino-prop-1-ene. J Chem Sci 125:939–948. doi:10.1007/s12039-013-0466-y
Fonseca TAO, Freitas MP, Cormanich RA, Ramalho TC, Tormena CF, Rittner R (2012) Computational evidence for intramolecular hydrogen bonding and nonbonding X···O interactions in 2′-haloflavonols, Beilstein. J Org Chem 8:112–117. doi:10.3762/bjoc.8.12
Garcia R, Perez R (2002) Dynamic atomic force microscopy methods. Surf Sci Rep 47:197–301. doi:10.1016/S0167-5729(02)00077-8
Grabowski SJ (2001) An estimation of strength of intramolecular hydrogen bonds—ab initio and AIM studies. J Mol Struct 562:137–143. doi:10.1016/S0022-2860(00)00863-2
Hallett AJ, Pope SJA (2011) Towards near-IR emissive rhenium tricarbonyl complexes: synthesis and characterisation of unusual 2,2-biquinoline complexes. Inorg Chem Commun 14:1606–1608. doi:10.1016/j.inoche.2011.06.021
Hasselmann GM, Meyer GJ (1999) Diffusion-limited interfacial electron transfer with large apparent driving forces. J Phys Chem B 103:7671–7675. doi:10.1021/jp992086s
Hernandez-Acevedo L, Arratia-Perez R (2004) SPIN-ORBIT effects metal-metal multiple bonded M2X2 halide complexes. J Chil Chem Soc 49:361–365. doi:10.4067/S0717-97072004000400017
Herzog W, Bronner C, Loffler S, He B, Kratzert D, Stalke D, Hauser A, Wenger OS (2013) Electron transfer between hydrogen-bonded pyridylphenols and a photoexcited Rhenium(I) complex. Chem Phys Chem 14:1168–1176. doi:10.1002/cphc.201201069
Interrante LV, Nelson GV (1968) Olefin-phosphine complexes of Manganese(I) and Rhenium(I). Inorg Chem 7:2059–2063. doi:10.1021/ic50068a021
Kaplanis M, Stamatakis G, Papakonstantinou VD, Paravatou-Petsotas M, Demopoulos CA, Mitsopoulou CA (2014) Re(I) tricarbonyl complex of 1,10-phenanthroline-5,6-dione: DNA binding, cytotoxicity, anti-inflammatory and anti-coagulant effects towards platelet activating factor. J Inorg Biochem 135:1–9. doi:10.1016/j.jinorgbio.2014.02.003
Kia R, Safari F (2016) Synthesis, spectral and structural characterization and computational studies of rhenium(I)-tricarbonyl nitrito complexes of 2,2-bipyridine and 2,9-dimethylphenanthroline ligands: π-accepting character of the diimine ligands. Inorg Chim Acta 453:357–368. doi:10.1016/j.ica.2016.08.041
Kim TY, Elliott ABS, Shaffer KJ, McAdam CJ, Gordon KC, Crowley JD (2013) Rhenium(I) complexes of readily functionalized bidentate pyridyl-1,2,3-triazole ‘‘click’’ ligands: A systematic synthetic, spectroscopic and computational study. Polyhedron 52:1391–1398. doi:10.1016/j.poly.2012.05.003
Kim Y, Vanhelmont FWM, Stern CL, Hupp JT (2001) Synthesis and photophysical properties of luminescent rhenium(I) and manganese(I) polypyridine complexes containing pendant1,3,4oxadiazole/ triarylamine assemblies. Inorg Chim Acta 318:53–60. doi:10.1016/S0020-1693(01)00400-5
Kirgan R, Simpson M, Moore C, Day J, Bui L, Tanner C, Rillema DP (2007) Synthesis, characterization, photophysical, and computational studies of Rhenium(I) tricarbonyl complexes containing the derivatives of bipyrazine. Inorg Chem 46:6464–6472. doi:10.1021/ic700512j
Kleij AW, Kuil M, Tooke DM, Lutz M, Spek AL, Reek JNH (2005a) ZnII–salphen complexes as versatile building blocks for the construction of supramolecular box assemblies. Chem Eur J 11:4743–4750. doi:10.1002/chem.200500227
Kleij AW, Tooke DM, Spek AL, Reek JNH (2005b) A convenient synthetic route for the preparation of nonsymmetric metallo-salphen complexes. Eur J Inorg Chem 2005:4626–4634. doi:10.1002/ejic.200500628
Kowalski K, Szczupak L, Berna T, Czerwieniec R (2015) Luminescent rhenium(I)echromone bioconjugate: Synthesis, photophysical properties, and confocal luminescence microscopy investigation. J Organomet Chem 782:124–130. doi:10.1016/j.jorganchem.2015.01.017
Lam ST, Zhu N, Ka-Man Au V, Wing-Wah Yam V (2015) Synthesis, characterization, electrochemistry and photophysical studies of rhenium(I) tricarbonyl diimine complexes with carboxaldehyde alkynyl ligands. Polyhedron 86:10–16. doi:10.1016/j.poly.2014.03.051
Leonidova A, Gasser G (2014) Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem Biol. 9:2180–2193. doi:10.1021/cb500528c
Lo KKW, Zhang KY, Li SPY (2011) Recent exploitation of luminescent Rhenium(I) tricarbonyl polypyridine complexes as biomolecular and cellular probes. Eur J Inorg Chem 24:3551–3568. doi:10.1002/ejic.201100469
Lopez R, Loeb B, Striplin D, Devenney M, Omberg K, Meyer TJ (2004) Tuning the excited states in fac-[Re(X2d ppz)(CO)3(L)]: intraligand, charge transfer or both? J Chil Chem Soc 49:149–155. doi:10.4067/S0717-97072004000200009
Losey DJ, Frenzel BA, Smith WM, Hightower SE, Hamaker CG (2013) Tricarbonyl rhenium complex of 2,6-bis(8′-quinolinyl)pyridine: synthesis, spectroscopic characterization, X-ray structure and DFT calculations. Inorg Chem Commun 30:46–48. doi:10.1016/j.inoche.2013.01.013
Machura B, Kruszynski R (2007) X-ray structure, spectroscopic characterisation and DFT calculations of the [Re(CO)3(2,2-biquinoline)Cl] complex. Polyhedron 26:3336–3342. doi:10.1016/j.poly.2007.03.014
Machura B, Świtlicka A, Nawrot I, Michalik K (2010a) Tricarbonyl rhenium complex of 2,2′-bis(4,5-dimethylimidazole), synthesis, spectroscopic characterization, X-ray structure and DFT calculations. Inorg Chem Commun 13:1317–1320. doi:10.1016/j.inoche.2010.07.025
Machura B, Wolff M, Gryca I (2010b) Novel rhenium(II) complex of 2,3,5,6-tetra(2-pyridyl)pyrazine: synthesis, X-ray studies, spectroscopic characterization and DFT calculations. Inorg Chem Commun 13:904–908. doi:10.1016/j.inoche.2010.04.022
Machura B, Switlicka A, Penkala M (2012) N- and S-bonded thiocyanate copper(II) complexes of 2,6-bis-(benzimidazolyl)pyridine—synthesis, spectroscopic characterization, X-ray structure and DFT calculations. Polyhedron 45:221–228. doi:10.1016/j.poly.2012.07.008
Machura B, Wolff M, Benoist E (2013a) Synthesis and spectroscopic characterization of novel oxido-bridged dinuclear rhenium(V) complex of 2-(aminomethyl)benzimidazole. X-ray crystal structures of [Re2O3Cl4(ambi)2]•CH3COCH3 and [Re2O3Cl4(ambi)2]•CH3CN. Inorg Chem Commun 29:101–105. doi:10.1016/j.inoche.2012.12.018
Machura B, Wolff M, Benoist E, Coulais Y (2013b) Tricarbonyl rhenium(I) complex of benzothiazole e Synthesis, spectroscopic characterization, X-ray crystal structure and DFT calculations. J Organomet Chem 724:82–87. doi:10.1016/j.jorganchem.2012.10.020
Mahmood A, Akgun Z, Peng Y, Müller P, Jiang Y, Berke H, Jones AG, Nicholson T (2013) The synthesis and characterization of rhenium nitrosyl complexes. The X-ray crystal structures of [ReBr2(NO)(NCMe)3], [Re(NO)(N5)](BPh4)2] and [ReBr2(NO)(NCMe) {py-CH2-NHCH2CH2 N(CH2-py)2}]. Inorg Chim Acta 405:455–460. doi:10.1016/j.ica.2013.01.017
Manbeck GF, Muckerman JT, Szalda DJ, Himeda Y, Fujita E (2015) Push or pull?Proton responsive ligand effects in rhenium tricarbonyl CO2 reduction catalysts. J Phys Chem B 24:7457–7466. doi:10.1021/jp511131x
Manicum AL, Visser HG, Engelbrecht I, Roodt A (2015) Crystal structure of fac-(acetylacetonato-k2 O,O')tricarbonyl(cyclohexyl-diphenylphosphinekP)rhenium(I),C26H28O5 PRe. Z Kristallogr NCS. 230:150–152. doi:10.1515/ncrs-2014-9013
Marake DT, Mokolokolo PP, Visser HG, Brinks A (2015) Structural comparison of group 7 tricarbonyl complexes of 2-{[2-(1H-imidazol-4-yl)ethyl]iminomethyl}-5-methylphenolate. Acta Cryst C71:423–429. doi:10.1107/S2053229615008360
Meyer GJ (2005) Molecular approaches to solar energy conversion with coordination compounds anchored to semiconductor surfaces. Inorg Chem 44:6852–6864. doi:10.1021/ic0505908
Meyer TJ, Caspar JV (1985) Photochemistry of metal–metal bonds. Chem Rev 85:187–218. doi:10.1021/cr00067a002
Muñoz-Castro A, Arratia-Perez R (2012) Spin–orbit effects on a gold-based superatom: a relativistic Jellium model. Phys Chem Chem Phys 14:1408–1411. doi:10.1039/C1CP22420D
Nagy PI (2014) Competing intramolecular vs. intermolecular hydrogen bonds in solution. Int J Mol Sci 15:19562–19633. doi:10.3390/ijms151119562
Partyka DV, Deligonul N, Washington MP, Gray TG (2009) fac-tricarbonyl Rhenium(I) azadipyrromethene complexes. Organometallics 28:5837–5840. doi:10.1021/om900552e
Pazik A, Kamińska B, Skwierawska A, Ponikiewski L (2016) Synthesis, structural and spectroscopic properties of asymmetric Schiff bases derived from 2,3-diaminopyridine. Chem Pap 70:1204. doi:10.1515/chempap-2016-0058
Pyykko P (1988) Relativistic effects in structural chemistry. Chem Rev 88:563–594. doi:10.1021/cr00085a006
Rabanal-Leon WA, Murillo-Lopez JA, Paez-Hernandez D, Arratia-perez R (2014) Understanding the influence of terminal ligands on the electronic structure and bonding nature in [Re6(μ3-Q8)]2+ clusters. J Phys Chem A 118:11083–11089. doi:10.1021/jp508892r
Rabanal-Leon WA, Murillo-Lopez JA, Paez-Hernadez D, Arratia-Perez R (2015) Exploring the nature of the excitation energies in [Re6(μ3-Q8)X6]4− clusters: a relativistic approach. Phys Chem Chem Phys 17:17611–17617. doi:10.1039/C5CP02003D
Ramírez-Tagle R, Alvarado-Soto L, Hernández-Acevedo L, Arratia-Pérez R (2010) Spin-Orbit and solvent effects in the luminescent [Re6Q8(NCS)6] −4 , Q=S, Se, Te clusters: molecular sensors and molecular devices. J Chil Chem Soc 55:39–43. doi:10.4067/S0717-97072010000100010
Robinson EA, Lister MW (1963) A linear relationship between bond orfers and stretching force contants. Can J Chem 41:2988–2995. doi:10.1139/v63-439
Sacksteder LA, Zipp AP, Brown EA, Streich J, Demas JN, DeGraf BA (1990) Luminescence studies of pyridine α-diimine Rhenium(I) tricarbonyl complexes. Inorg Chem 29:4335–4340. doi:10.1021/ic00346a033
SHELXL-PC Package (1988) Bruker analytical X-ray systems, Madison
Sreejith SS, Mohan N, Aiswarya N, Prathapachandra Kurup MR (2016) Inclusion, pseudo-inclusion compounds and coordination polymer of Pd(II), Zn(II) and Cd(II) from salen-type Schiff base ligand with a 1,3-diimino spacer group: crystal structures, spectroscopic and thermal studies. Polyhedron 115:180–192. doi:10.1016/j.poly.2016.05.007
Thorp-Greenwood FL (2012) An introduction to organometallic complexes in fluorescence cell imaging: current applications and future prospects. Organometallics 31:5686–5692. doi:10.1021/om3004477
Thorp-Greenwood FL, Balasingham RG, Coogan MP (2012) Organometallic complexes of transition metals in luminescent cell imaging applications. J Organomet Chem 714:12–21. doi:10.1016/j.jorganchem.2012.01.020
Toganoh M, Ikeda S, Furuta H (2007) Synthesis, reactivity, and properties of N-fused porphyrin Rhenium(I) tricarbonyl complexes. Inorg Chem 46:10003–10015. doi:10.1021/ic701208g
Wallace L, Rillema DP (1993) Photophysical properties of Rhenium(I) tricarbonyl complexes containing alkyl- and aryl-substituted phenanthrolines as ligands. Inorg Chem 32:3836–3843. doi:10.1021/ic00070a012
Wang W, Andy Hor TS, Yan YK (2006) Synthesis and X-ray structures of rhenium(I) carbonyl aminoalkoxide and aminocarboxylate complexes. Inorg Chim Acta 359:3754–3762. doi:10.1016/j.ica.2006.03.027
Wei L, Babich JW, Ouellette W, Zubieta J (2006) Developing the {M(CO)3}+ core for fluorescence applications: rhenium tricarbonyl core complexes with benzimidazole, quinoline, and tryptophan derivatives. Inorg Chem 45:3057–3066. doi:10.1021/ic0517319
Wenger OS (2015) Proton-coupled electron transfer with photoexcited ruthenium(II), rhenium(I), and iridium(III) complexes. Coord Chem Rev 282:150–158. doi:10.1016/j.ccr.2014.03.025
Wenger OS, Henling LM, Day MW, Winkler JR, Gray HB (2004) Photoswitchable luminescence of Rhenium(I) tricarbonyl diimines. Inorg Chem 43:2043–2048. doi:10.1021/ic030324z
Winslow LN, Rillema DP, Welch JH, Singh P (1989) A Rhenium (I) bipyrimidine tricarbonyl complex containing methyl viologen as the sixth ligand: NMR and structural results. Inorg Chem 28:1596–1599. doi:10.1021/ic00307a034
Wrighton M, Morse DL (1974) The nature of the lowest excited state in tricarbonylchloro- 1, 10-phenanthrolinerhenium(I) and related complexes. J Am Chem Soc 4:998–1003. doi:10.1021/ja00811a008
Yue Y, Grusenmeyer T, Ma Z, Zhang P, Pham TT, Mague JT, Donahue JP, Schmehl RH, Beratan DN, Rubtsov IV (2013) Evaluating the extent of intramolecular charge transfer in the excited states of Rhenium(I) donor–acceptor complexes with time-resolved vibrational spectroscopy. J Phys Chem B 117:15903–15916. doi:10.1021/jp409628e
Zhang MT, Irebo T, Johansson O, Hammarstrom L (2011) Proton-coupled electron transfer from tyrosine: a strong rate dependence on intramolecular proton transfer distance. J Am Chem Soc 133:13224–13227. doi:10.1021/ja203483j
Acknowledgements
Funded partially by project RC120001 of the Iniciativa Científica Milenio del Ministerio de Economía, Fomento y Turismo del Gobierno de Chile and Nucleo UNAB DI-1419-16/N; E. Molins acknowledges funding by the Spanish Ministerio de Economía y Competividad (ENE2015-63969 and SEV2015-0496). We thank Dr. Dayan Paez-Hernandez (UNAB) for relativistic DFT calculations; Dr. Ivonne Chavez (Departamento de Química Inorgánica, Pontificia Universidad Católica de Chile) for instrumentals facilities, Dr. Juan A. Fuentes (Laboratorio de Genética y Patogénesis Bacteriana, Facultad de Ciencias Biológicas, UNAB) for biological contributions and B.A. Alfonso Inzunza G. for his help with the English translation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11696_2017_196_MOESM1_ESM.docx
Supporting Information: CCDC 1448327 contains the supplementary crystallographic data for C2. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. (DOCX 522 kb)
Rights and permissions
About this article
Cite this article
Carreño, A., Gacitúa, M., Molins, E. et al. X-ray diffraction and relativistic DFT studies on the molecular biomarker fac-Re(CO)3(4,4′-dimethyl-2,2′-bpy)(E-2-((3-amino-pyridin-4-ylimino)-methyl)-4,6-di-tert-butylphenol)(PF6). Chem. Pap. 71, 2011–2022 (2017). https://doi.org/10.1007/s11696-017-0196-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0196-6