Abstract
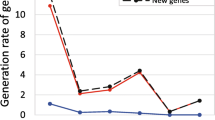
We analyze the global structure and evolution of human gene coexpression networks driven by new gene integration. When the Pearson correlation coefficient is greater than or equal to 0.5, we find that the coexpression network consists of 334 small components and one “giant” connected subnet comprising of 6317 interacting genes. This network shows the properties of power-law degree distribution and small-world. The average clustering coefficient of younger genes is larger than that of the elderly genes (0.6685 vs. 0.5762). Particularly, we find that the younger genes with a larger degree also show a property of hierarchical architecture. The younger genes play an important role in the overall pivotability of the network and this network contains few redundant duplicate genes. Moreover, we find that gene duplication and orphan genes are two dominant evolutionary forces in shaping this network. Both the duplicate genes and orphan genes develop new links through a “rich-gets-richer” mechanism. With the gradual integration of new genes into the ancestral network, most of the topological structure features of the network would gradually increase. However, the exponent of degree distribution and modularity coefficient of the whole network do not change significantly, which implies that the evolution of coexpression networks maintains the hierarchical and modular structures in human ancestors.
Similar content being viewed by others
References
Albert, R., Jeong, H., and Barabási, A.L. (2000). Error and attack tolerance of complex networks. Nature 406, 378–382.
Barkai, N., and Leibler, S. (1997). Robustness in simple biochemical networks. Nature 387, 913–917.
Barabási, A.L., and Albert, R. (1999). Emergence of scaling in random networks. Science 286, 509–512.
Barabási, A.L., and Oltvai, Z.N. (2004). Network biology: understanding the cell’s functional organization. Nat Rev Genet 5, 101–113.
Chung, F., Lu, L., Dewey, T.G., and Galas, D.J. (2003). Duplication models for biological networks. J Comput Biol 10, 677–687.
Cohen, R., and Havlin, S. (2003). Scale-free networks are ultrasmall. Phys Rev Lett 90, 058701.
Clauset, A., Newman, M.E.J., and Moore, C. (2004). Finding community structure in very large networks. Phys Rev E 70, 066111.
Chung, W.Y., Albert, R., Albert, I., Nekrutenko, A., and Makova, K.D. (2006). Rapid and asymmetric divergence of duplicate genes in the human gene coexpression network. BMC Bioinformatics 7, 46, 1–14.
Crombach, A., and Hogeweg, P. (2008). Evolution of evolvability in gene regulatory networks. PLoS Comput Biol 4, e1000112.
Chen, S., Krinsky, B.H., and Long, M. (2013). New genes as drivers of phenotypic evolution. Nat Rev Genet 14, 645–660.
Girvan, M., and Newman, M.E.J. (2002). Community structure in social and biological networks. Proc Natl Acad Sci USA 99, 7821–7826.
Horvath, S., and Dong, J. (2008). Geometric interpretation of gene coexpression network analysis. PLoS Comput Biol 4, e1000117.
Hedges, S.B., Marin, J., Suleski, M., Paymer, M., and Kumar, S. (2015). Tree of life reveals clock-like speciation and diversification. Mol Biol Evol 32, 835–845.
Jeong, H., Tombor, B., Albert, R., Oltvai, Z.N., and Barabási, A.L. (2000). The large-scale organization of metabolic networks. Nature 407, 651–654.
Jordan, I.K., Marino-Ramirez, L., Wolf, Y.I., and Koonin, E.V. (2004). Conservation and coevolution in the scale-free human gene coexpression network. Mol Biol Evol 21, 2058–2070.
Lee, H.K., Hsu, A.K., Sajdak, J., Qin, J., and Pavlidis, P. (2004). Coexpression analysis of human genes across many microarray data sets. Genome Res 14, 1085–1094.
Li, M., Li, Q., Ganegoda, G.U., Wang, J.X., Wu, F.X., and Pan, Y. (2014). Prioritization of orphan disease-causing genes using topological feature and GO similarity between proteins in interaction networks. Sci China Life Sci 57, 1064–1071.
Maslov, S., and Sneppen, K. (2002). Specificity and stability in topology of protein networks. Science 296, 910–913.
Oldham, M.C., Horvath, S., and Geschwind, D.H. (2006). Conservation and evolution of gene coexpression networks in human and chimpanzee brains. Proc Natl Acad Sci USA 103, 17973–17978.
Obayashi, T., Okamura, Y., Ito, S., Tadaka, S., Motoike, I.N., and Kinoshita, K. (2012). COXPRESdb: a database of comparative gene coexpression networks of eleven species for mammals. Nucleic Acids Res 41, D1014–D1020.
Pastor-Satorras, R., Smith, E., and Solé, R.V. (2003). Evolving protein interaction networks through gene duplication. J Theor Biol 222, 199–210.
Prieto, C., Risueño, A., Fontanillo, C., and De las Rivas, J. (2008). Human gene coexpression landscape: confident network derived from tissue transcriptomic profiles. PLoS ONE 3, e3911.
Ravasz, E., Somera, A.L., Mongru, D.A., Oltvai, Z.N., and Barabási, A.L. (2002). Hierarchical organization of modularity in metabolic networks. Science 297, 1551–1555.
Shen-Orr, S.S., Milo, R., Mangan, S., and Alon, U. (2002). Network motifs in the transcriptional regulation network of Escherichia coli. Nat Genet 31, 64–68.
Sorrells, T.R., and Johnson, A.D. (2015). Making sense of transcription networks. Cell 161, 714–723.
Tautz, D., and Domazet-Lošo, T. (2011). The evolutionary origin of orphan genes. Nat Rev Genet 12, 692–702.
Watts, D.J., and Strogatz, S.H. (1998). Collective dynamics of ‘small-world’ networks. Nature 393, 440–442.
Wagner, A. (2001). The yeast protein interaction network evolves rapidly and contains few redundant duplicate genes. Mol Biol Evol 18, 1283–1292.
Wagner, A. (2003). How the global structure of protein interaction networks evolves. Proc R Soc London Ser B-Biol Sci 270, 457–466.
Yu, H., Mitra, R., Yang, J., Li, Y.Y., and Zhao, Z.M. (2014). Algorithms for network-based identification of differential regulators from transcriptome data: a systematic evaluation. Sci China Life Sci 57, 1090–1102.
Zhang, Y.F., Zhang, R., and Su, B. (2009). Diversity and evolution of microRNA gene clusters. Sci China Ser C-Life Sci 52, 261–266.
Zhang, Y.E., Vibranovski, M.D., Landback, P., Marais, G.A.B., and Long, M. (2010). Chromosomal redistribution of male-biased genes in mammalian evolution with two bursts of gene gain on the X chromosome. PLoS Biol 8, e1000494.
Zhang, Y.E., Landback, P., Vibranovski, M.D., and Long, M. (2011). Accelerated recruitment of new brain development genes into the human genome. PLoS Biol 9, e1001179.
Zhang, W., Landback, P., Gschwend, A.R., Shen, B., and Long, M. (2015). New genes drive the evolution of gene interaction networks in the human and mouse genomes. Genome Biol 16, 202.
Acknowledgements
We thank Profs. Yicang Zhou and Yanni Xiao for their valuable discussion. This work was supported by grants from the National Natural Science Foundation of China (11571272, 11201368 and 11631012), the National Science and Technology Major Project of China (2012ZX10002001), the Natural Science Foundation of Shaanxi Province (2015JQ1011) and the China Postdoctoral Science Foundation (2014M560755).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zu, J., Gu, Y., Li, Y. et al. Topological evolution of coexpression networks by new gene integration maintains the hierarchical and modular structures in human ancestors. Sci. China Life Sci. 62, 594–608 (2019). https://doi.org/10.1007/s11427-019-9483-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-9483-6