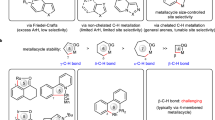
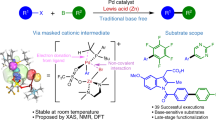
Abstract
Ni−Al bimetallic catalysis proves to be an efficient catalytic strategy for unreactive bond transformations. Recently, chiral bifunctional ligands, especially amphoteric secondary phosphine oxide (SPO) ligand, are used for a more powerful synergistic effect in the bimetal-catalyzed reactions, providing not only milder reaction conditions and higher reactivity but also excellent reaction selectivity. Herein, we give a brief review on the development of Ni−Al bimetallic catalytic system and highlight recent advances in enantioselective Ni−Al bimetallic catalysis for unreactive bond transformation.
Similar content being viewed by others
References
(a) D’Souza DM, Müller TJJ. Chem Soc Rev. 2007, 36: 1095–1108
Das S, Brudvig GW, Crabtree RH. Chem Commun. 2008, 127: 413–424
Diez-Gonzalez S, Marion N, Nolan SP. Chem Rev. 2009, 109: 3612–3676
Zhong C, Shi X. Eur J Org Chem. 2010, 2010: 2999–3025
Du Z, Shao Z. Chem Soc Rev. 2013, 42: 1337–1378
Hu Y, Wang C. Sci China Chem. 2016, 59: 1301–1305
Li X, He X, Liu X, He LN. Sci China Chem. 2017, 60: 841–852
(a) Goossen LJ, Goossen K, Stanciu C. Angew Chem Int Ed. 2009, 48: 3569–3571
Su B, Cao ZC, Shi ZJ. Acc Chem Res. 2015, 48: 886–896
Wang Q, Su Y, Li L, Huang H. Chem Soc Rev. 2016, 45: 1257–1272
Tobisu M, Chatani N. Acc Chem Res. 2015, 48: 1717–1726
Gao Y, Ji CL, Hong X. Sci China Chem. 2017, 60: 1413–1424
Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010, 110: 624–655
Labinger JA, Bercaw JE. Nature. 2002, 417: 507–514
Wencel-Delord J, Dröge T, Liu F, Glorius F. Chem Soc Rev. 2011, 40: 4740–4761
(a) Rousseau G, Breit B. Angew Chem Int Ed. 2011, 50: 2450–2494
Rouquet G, Chatani N. Angew Chem Int Ed. 2013, 52: 11726–11743
Corbet M, De Campo F. Angew Chem Int Ed. 2013, 52: 9896–9898
Song G, Li X. Acc Chem Res. 2015, 48: 1007–1020
Zhu RY, Farmer ME, Chen YQ, Yu JQ. Angew Chem Int Ed. 2016, 55: 10578–10599
He J, Wasa M, Chan KSL, Shao Q, Yu JQ. Chem Rev. 2017, 117: 8754–8786
(a) Lyons TW, Sanford MS. Chem Rev. 2010, 110: 1147–1169
Engle KM, Yu JQ. J Org Chem. 2013, 78: 8927–8955
Ye B, Cramer N. Acc Chem Res. 2015, 48: 1308–1318
Saint-Denis TG, Zhu RY, Chen G, Wu QF, Yu JQ. Science. 2018, 359: eaao4798
(a) Biswas J, Maxwell IE. Appl Catal. 1990, 63: 197–258
Otterstedt JE, Gevert SB, Jäås SG, Menon PG. Appl Catal. 1986, 22: 159–179
(a) Fu J, Huo X, Li B, Zhang W. Org Biomol Chem. 2017, 15: 9747–9759
Pye DR, Mankad NP. Chem Sci. 2017, 8: 1705–1718
Mankad NP. Chem Eur J. 2016, 22: 5822–5829
Hetterscheid DGH, Chikkali SH, de Bruin B, Reek JNH. ChemCatChem. 2013, 5: 2785–2793
Park J, Hong S. Chem Soc Rev. 2012, 41: 6931–6943
Pérez-Temprano MH, Casares JA, Espinet P. Chem Eur J. 2012, 18: 1864–1884
Matsunaga S, Shibasaki M. Bull Chem Soc Jpn. 2008, 81: 60–75
van den Beuken EK, Feringa BL. Tetrahedron. 1998, 54: 12985–13011
Rowlands GJ. Tetrahedron. 2001, 57: 1865–1882
(a) Trost BM, Toste FD, Pinkerton AB. Chem Rev. 2001, 101: 2067–2096
Bolm C, Legros J, Le Paih J, Zani L. Chem Rev. 2004, 104: 6217–6254
Yin L, Liebscher J. Chem Rev. 2007, 107: 133–173
Monnier F, Taillefer M. Angew Chem Int Ed. 2009, 48: 6954–6971
Rodríguez N, Goossen LJ. Chem Soc Rev. 2011, 40: 5030–5048
Yeung CS, Dong VM. Chem Rev. 2011, 111: 1215–1292
(a) Jun CH. Chem Soc Rev. 2004, 33: 610–618
Murakami M, Matsuda T. Chem Commun. 2011, 47: 1100–1105
Dermenci A, Coe JW, Dong G. Org Chem Front. 2014, 1: 567–581
Souillart L, Cramer N. Chem Rev. 2015, 115: 9410–9464
Murakami M, Ishida N. J Am Chem Soc. 2016, 138: 13759–13769
Chen P, Billett BA, Tsukamoto T, Dong G. ACS Catal. 2017, 7: 1340–1360
Fumagalli G, Stanton S, Bower JF. Chem Rev. 2017, 117: 9404–9432
Chen F, Wang T, Jiao N. Chem Rev. 2014, 114: 8613–8661
(a) Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007, 107: 3117–3179
Seiser T, Cramer N. Org Biomol Chem. 2009, 7: 2835–2840
Tipper CFH. J Chem Soc. 1955, 2045–2046
Wiberg KB, Fenoglio RA. J Am Chem Soc. 1968, 90: 3395–3397
(a) William Suggs J, Cox SD. J Organomet Chem. 1981, 221: 199–201
Suggs JW, Jun CH. J Am Chem Soc. 1984, 106: 3054–3056
Jun CH, Lee H. J Am Chem Soc. 1999, 121: 880–881
Jun CH, Lee H, Lim SG. J Am Chem Soc. 2001, 123: 751–752
Dreis AM, Douglas CJ. J Am Chem Soc. 2009, 131: 412–413
Wang J, Chen W, Zuo S, Liu L, Zhang X, Wang J. Angew Chem Int Ed. 2012, 51: 12334–12338
(a) Tobisu M, Chatani N. Chem Soc Rev. 2008, 37: 300–307
Kou X, Fan J, Tong X, Shen Z. Chin J Org Chem. 2013, 33: 1407
Chen F, Wang T, Jiao N. Chem Rev. 2014, 114: 8613–8661
Wen Q, Lu P, Wang Y. RSC Adv. 2014, 4: 47806–47826
Murahashi S, Naota T, Nakajima N. J Org Chem. 1986, 51: 898–901
Taw FL, White PS, Bergman RG, Brookhart M. J Am Chem Soc. 2002, 124: 4192–4193
Nakao Y, Oda S, Hiyama T. J Am Chem Soc. 2004, 126: 13904–13905
Nakao Y, Yukawa T, Hirata Y, Oda S, Satoh J, Hiyama T. J Am Chem Soc. 2006, 128: 7116–7117
Tobisu M, Kita Y, Chatani N. J Am Chem Soc. 2006, 128: 8152–8153
(a) DuPont. Chem Eng News. 1971, 49: 30–31
Huthmacher K, Krill S. In: Cornils B, Hermann WA, eds. Applied Homogeneous Catalysis with Organometallic Compounds. 2nd ed. Weinheim: Wiley-VCH. 2002
(a) Nakao Y, Hiyama T. J Syn Org Chem Jpn. 2007, 65: 999–1008
Nakao Y, Hiyama T. Pure Appl Chem. 2008, 80: 1097–1107
Yada A, Yukawa T, Idei H, Nakao Y, Hiyama T. Bull Chem Soc Jpn. 2010, 83: 619–634
Nakao Y. Bull Chem Soc Jpn. 2012, 85: 731–745
Brunkan NM, Brestensky DM, Jones WD. J Am Chem Soc. 2004, 126: 3627–3641
Nakao Y, Hirata Y, Tanaka M, Hiyama T. Angew Chem Int Ed. 2008, 47: 385–387
Watson MP, Jacobsen EN. J Am Chem Soc. 2008, 130: 12594–12595
Hirata Y, Yada A, Morita E, Nakao Y, Hiyama T, Ohashi M, Ogoshi S. J Am Chem Soc. 2010, 132: 10070–10077
Minami Y, Yoshiyasu H, Nakao Y, Hiyama T. Angew Chem Int Ed. 2013, 52: 883–887
Miyazaki Y, Ohta N, Semba K, Nakao Y. J Am Chem Soc. 2014, 136: 3732–3735
Rondla NR, Ogilvie JM, Pan Z, Douglas CJ. Chem Commun. 2014, 50: 8974–8977
Nakao Y, Yada A, Ebata S, Hiyama T. J Am Chem Soc. 2007, 129: 2428–2429
Nakao Y, Ebata S, Yada A, Hiyama T, Ikawa M, Ogoshi S. J Am Chem Soc. 2008, 130: 12874–12875
(a) Hirata Y, Yukawa T, Kashihara N, Nakao Y, Hiyama T. J Am Chem Soc. 2009, 131: 10964–10973
Yada A, Yukawa T, Nakao Y, Hiyama T. Chem Commun. 2009, 107: 3931–3933
Nakao Y, Yada A, Hiyama T. J Am Chem Soc. 2010, 132: 10024–10026
Yada A, Ebata S, Idei H, Zhang D, Nakao Y, Hiyama T. Bull Chem Soc Jpn. 2010, 83: 1170–1184
Yamada Y, Ebata S, Hiyama T, Nakao Y. Tetrahedron. 2015, 71: 4413–4417
(a) Huang J, Haar CM, Nolan SP, Marcone JE, Moloy KG. Organometallics. 1999, 18: 297–299
Shen Q, Hartwig JF. J Am Chem Soc. 2007, 129: 7734–7735
Nakai K, Kurahashi T, Matsubara S. J Am Chem Soc. 2011, 133: 11066–11068
(a) Nakai K, Kurahashi T, Matsubara S. Org Lett. 2013, 15: 856–859
Nakai K, Kurahashi T, Matsubara S. Tetrahedron. 2015, 71: 4512–4517
(a) Patra T, Agasti S, Akanksha S, Maiti D. Chem Commun. 2013, 49: 69–71
Patra T, Agasti S, Modak A, Maiti D. Chem Commun. 2013, 49: 8362–8364
Romeder G. Hydrogen Cyanide. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2000
(a) Fang X, Yu P, Morandi B. Science. 2016, 351: 832–836
Yu P, Morandi B. Angew Chem Int Ed. 2017, 56: 15693–15697
Fang X, Yu P, Prina Cerai G, Morandi B. Chem Eur J. 2016, 22: 15629–15633
Tamaki T, Ohashi M, Ogoshi S. Angew Chem Int Ed. 2011, 50: 12067–12070
(a) Nakao Y, Kanyiva KS, Hiyama T. J Am Chem Soc. 2008, 130: 2448–2449
Yang L, Semba K, Nakao Y. Angew Chem Int Ed. 2017, 56: 4853–4857
Hara N, Saito T, Semba K, Kuriakose N, Zheng H, Sakaki S, Nakao Y. J Am Chem Soc. 2018, 140: 7070–7073
(a) Nakao Y, Idei H, Kanyiva KS, Hiyama T. J Am Chem Soc. 2009, 131: 5070–5071
Kanyiva KS, Löbermann F, Nakao Y, Hiyama T. Tetrahedron Lett. 2009, 50: 3463–3466
Nakao Y, Idei H, Kanyiva KS, Hiyama T. J Am Chem Soc. 2009, 131: 15996–15997
Nakao Y, Yamada Y, Kashihara N, Hiyama T. J Am Chem Soc. 2010, 132: 13666–13668
Tsai CC, Shih WC, Fang CH, Li CY, Ong TG,Ya. GPA. J Am Chem Soc. 2010, 132: 11887–11889
Nakao Y, Morita E, Idei H, Hiyama T. J Am Chem Soc. 2011, 133: 3264–3267
Miyazaki Y, Yamada Y, Nakao Y, Hiyama T. Chem Lett. 2012, 41: 298–300
Shih WC, Chen WC, Lai YC, Yu MS, Ho JJ, Yap GPA, Ong TG. Org Lett. 2012, 14: 2046–2049
Tamura R, Yamada Y, Nakao Y, Hiyama T. Angew Chem. 2012, 124: 5777–5780
Liu S, Sawicki J, Driver TG. Org Lett. 2012, 14: 3744–3747
Lee WC, Wang CH, Lin YH, Shih WC, Ong TG. Org Lett. 2013, 15: 5358–5361
Yu MS, Lee WC, Chen CH, Tsai FY, Ong TG. Org Lett. 2014, 16: 4826–4829
Lee WC, Shih WC, Wang TH, Liu Y, Yap GPA, Ong TG. Tetrahedron. 2015, 71: 4460–4464
Lee WC, Chen CH, Liu CY, Yu MS, Lin YH, Ong TG. Chem Commun. 2015, 51: 17104–17107
Okumura S, Tang S, Saito T, Semba K, Sakaki S, Nakao Y. J Am Chem Soc. 2016, 138: 14699–14704
Okumura S, Nakao Y. Org Lett. 2017, 19: 584–587
Inoue F, Saito T, Semba K, Nakao Y. Chem Commun. 2017, 53: 4497–4500
Okumura S, Komine T, Shigeki E, Semba K, Nakao Y. Angew Chem Int Ed. 2018, 57: 929–932
Donets PA, Cramer N. Angew Chem. 2015, 127: 643–647
Miura T, Yamauchi M, Murakami M. Chem Commun. 2009, 36: 1470–1471
Kajita Y, Matsubara S, Kurahashi T. J Am Chem Soc. 2008, 130: 6058–6059
Shiba T, Kurahashi T, Matsubara S. J Am Chem Soc. 2013, 135: 13636–13639
Nakai K, Kurahashi T, Matsubara S. Chem Lett. 2013, 42: 1238–1240
(a) Sergeev AG, Hartwig JF. Science. 2011, 332: 439–443
Sergeev AG, Webb JD, Hartwig JF. J Am Chem Soc. 2012, 134: 20226–20229
(a) Hsieh JC, Ebata S, Nakao Y, Hiyama T. Synlett. 2010, 11: 1709–1711
Watson MP, Jacobsen EN. J Am Chem Soc. 2008, 130: 12594–12595
Diesel J, Finogenova AM, Cramer N. J Am Chem Soc. 2018, 140: 4489–4493
(a) Yoshikai N, Mashima H, Nakamura E. J Am Chem Soc. 2005, 127: 17978–17979
Yoshikai N, Matsuda H, Nakamura E. J Am Chem Soc. 2009, 131: 9590–9599
Ackermann L, Althammer A. Chem Unserer Zeit. 2009, 43: 74–83
Jin Z, Li YJ, Ma YQ, Qiu LL, Fang JX. Chem Eur J. 2012, 18: 446–450
For related reviews on SPO ligands, see: (a) Dubrovina NV, Börner A. Angew Chem Int Ed. 2004, 43: 5883–5886
Ackermann L, Born R, Spatz JH, Althammer A, Gschrei CJ. Pure Appl Chem. 2006, 78: 209–214
Nemoto T, Hamada Y. Chem Record. 2007, 7: 150–158
Nemoto T. Chem Pharm Bull. 2008, 56: 1213–1228
Ackermann L. Isr J Chem. 2010, 50: 652–663
Nemoto T, Hamada Y. Tetrahedron. 2011, 67: 667–687
Shaikh TM, Weng CM, Hong FE. Coordin Chem Rev. 2012, 256: 771–803
For recent enantioselective examples, see: (h) Achard T. Chimia. 2016, 70: 8–19
Dong K, Wang Z, Ding K. J Am Chem Soc. 2012, 134: 12474–12477
Dong K, Li Y, Wang Z, Ding K. Angew Chem Int Ed. 2013, 52: 14191–14195
Chen C, Zhang Z, Jin S, Fan X, Geng M, Zhou Y, Wen S, Wang X, Chung LW, Dong XQ, Zhang X. Angew Chem Int Ed. 2017, 56: 6808–6812
Donets PA, Cramer N. J Am Chem Soc. 2013, 135: 11772–11775
Liu QS, Wang DY, Yang ZJ, Luan YX, Yang JF, Li JF, Pu YG, Ye M. J Am Chem Soc. 2017, 139: 18150–18153
Wang YX, Qi SL, Luan YX, Han XW, Wang S, Chen H, Ye M. J Am Chem Soc. 2018, 140: 5360–5364
Tan KL, Bergman RG, Ellman JA. J Am Chem Soc. 2001, 123: 2685–2686
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21672107) and the “1000-Youth Talents Plan”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, YX., Ye, M. Recent advances in Ni−Al bimetallic catalysis for unreactive bond transformation. Sci. China Chem. 61, 1004–1013 (2018). https://doi.org/10.1007/s11426-018-9333-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-018-9333-x