Abstract
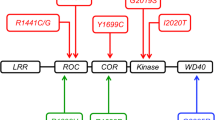
Mutations in the Leucine-rich repeat kinase 2 (LRRK2) gene have been implicated in the pathogenesis of Parkinson’s disease (PD). Identification of PD-associated LRRK2 mutations has led to the development of novel animal models, primarily in mice. However, the characteristics of human LRRK2 and mouse Lrrk2 protein have not previously been directly compared. Here we show that proteins from different species have different biochemical properties, with the mouse protein being more stable but having significantly lower kinase activity compared to the human orthologue. In examining the effects of PD-associated mutations and risk factors on protein function, we found that conserved substitutions such as G2019S affect human and mouse LRRK2 proteins similarly, but variation around position 2385, which is not fully conserved between humans and mice, induces divergent in vitro behavior. Overall our results indicate that structural differences between human and mouse LRRK2 are likely responsible for the different properties we have observed for these two species of LRRK2 protein. These results have implications for disease modelling of LRRK2 mutations in mice and on the testing of pharmacological therapies in animals.







Similar content being viewed by others
References
Kumaran R, Cookson MR (2015) Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson’s disease. Hum Mol Genet 24:R32–R44. https://doi.org/10.1093/hmg/ddv236
Zimprich A, Biskup S, Leitner P et al (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44:601–607. https://doi.org/10.1016/j.neuron.2004.11.005
Paisán-Ruíz C, Jain S, Evans EW et al (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44:595–600. https://doi.org/10.1016/j.neuron.2004.10.023
Funayama M, Hasegawa K, Ohta E et al (2005) An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann Neurol 57:918–921. https://doi.org/10.1002/ana.20484
Hernandez DG, Reed X, Singleton AB (2016) Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem 139(Suppl 1):59–74. https://doi.org/10.1111/jnc.13593
Cookson MR (2010) The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci 11:791–797. https://doi.org/10.1038/nrn2935
Greggio E, Jain S, Kingsbury A et al (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 23:329–341. https://doi.org/10.1016/j.nbd.2006.04.001
Skibinski G, Nakamura K, Cookson MR, Finkbeiner S (2014) Mutant LRRK2 toxicity in neurons depends on LRRK2 levels and synuclein but not kinase activity or inclusion bodies. J Neurosci 34:418–433. https://doi.org/10.1523/JNEUROSCI.2712-13.2014
Lee BD, Shin J-H, VanKampen J et al (2010) Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat Med 16:998–1000. https://doi.org/10.1038/nm.2199
Yao C, Johnson WM, Gao Y et al (2013) Kinase inhibitors arrest neurodegeneration in cell and C. elegans models of LRRK2 toxicity. Hum Mol Genet 22:328–344. https://doi.org/10.1093/hmg/dds431
Liu Z, Hamamichi S, Lee BD et al (2011) Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson’s disease models. Hum Mol Genet 20:3933–3942. https://doi.org/10.1093/hmg/ddr312
Li T, He X, Thomas JM et al (2015) A novel GTP-binding inhibitor, FX2149, attenuates LRRK2 toxicity in Parkinson’s disease models. PloS One 10:e0122461. https://doi.org/10.1371/journal.pone.0122461
West AB (2015) Ten years and counting: moving leucine-rich repeat kinase 2 inhibitors to the clinic. Mov Disord 30:180–189. https://doi.org/10.1002/mds.26075
Rudenko IN, Kaganovich A, Hauser DN et al (2012) The G2385R variant of leucine-rich repeat kinase 2 associated with Parkinson’s disease is a partial loss-of-function mutation. Biochem J 446:99–111. https://doi.org/10.1042/BJ20120637
Rudenko IN, Chia R, Cookson MR (2012) Is inhibition of kinase activity the only therapeutic strategy for LRRK2-associated Parkinson’s disease? BMC Med 10:20. https://doi.org/10.1186/1741-7015-10-20
Langston RG, Rudenko IN, Cookson MR (2016) The function of orthologues of the human Parkinson’s disease gene LRRK2 across species: implications for disease modelling in preclinical research. Biochem J 473:221–232. https://doi.org/10.1042/BJ20150985
Yue M, Hinkle KM, Davies P et al (2015) Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol Dis 78:172–195. https://doi.org/10.1016/j.nbd.2015.02.031
Tong Y, Pisani A, Martella G et al (2009) R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci USA 106:14622–14627. https://doi.org/10.1073/pnas.0906334106
Greggio E, Cookson MR (2009) Leucine-rich repeat kinase 2 mutations and Parkinson’s disease: three questions. ASN Neuro. https://doi.org/10.1042/AN20090007
West AB, Moore DJ, Biskup S et al (2005) Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA 102:16842–16847. https://doi.org/10.1073/pnas.0507360102
Chia R, Haddock S, Beilina A et al (2014) Phosphorylation of LRRK2 by casein kinase 1α regulates trans-Golgi clustering via differential interaction with ARHGEF7. Nat Commun 5:5827. https://doi.org/10.1038/ncomms6827
Beilina A, Rudenko IN, Kaganovich A et al (2014) Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci USA 111:2626–2631. https://doi.org/10.1073/pnas.1318306111
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Nichols RJ, Dzamko N, Hutti JE et al (2009) Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson’s disease. Biochem J 424:47–60. https://doi.org/10.1042/BJ20091035
Rudenko IN, Kaganovich A, Langston RG et al (2017) The G2385R risk factor for Parkinson’s disease enhances CHIP-dependent intracellular degradation of LRRK2. Biochem J 474:1547–1558. https://doi.org/10.1042/BCJ20160909
Steger M, Tonelli F, Ito G et al (2016) Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife. https://doi.org/10.7554/eLife.12813
Ding X, Goldberg MS (2009) Regulation of LRRK2 stability by the E3 ubiquitin ligase CHIP. PLoS ONE 4:e5949. https://doi.org/10.1371/journal.pone.0005949
Nichols RJ, Dzamko N, Morrice NA et al (2010) 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem J 430:393–404. https://doi.org/10.1042/BJ20100483
Wang L, Xie C, Greggio E et al (2008) The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J Neurosci 28:3384–3391. https://doi.org/10.1523/JNEUROSCI.0185-08.2008
Jorgensen ND, Peng Y, Ho CC-Y et al (2009) The WD40 domain is required for LRRK2 neurotoxicity. PLoS ONE 4:e8463. https://doi.org/10.1371/journal.pone.0008463
Greggio E, Zambrano I, Kaganovich A et al (2008) The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem 283:16906–16914. https://doi.org/10.1074/jbc.M708718200
Klein CL, Rovelli G, Springer W et al (2009) Homo- and heterodimerization of ROCO kinases: LRRK2 kinase inhibition by the LRRK2 ROCO fragment. J Neurochem 111:703–715. https://doi.org/10.1111/j.1471-4159.2009.06358.x
Zhao J, Molitor TP, Langston JW, Nichols RJ (2015) LRRK2 dephosphorylation increases its ubiquitination. Biochem J 469:107–120. https://doi.org/10.1042/BJ20141305
Ito G, Okai T, Fujino G et al (2007) GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry 46:1380–1388. https://doi.org/10.1021/bi061960m
West AB, Moore DJ, Choi C et al (2007) Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet 16:223–232. https://doi.org/10.1093/hmg/ddl471
West AB, Cowell RM, Daher JPL et al (2014) Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J Comp Neurol 522:2465–2480. https://doi.org/10.1002/cne.23583
Acknowledgements
We are grateful to Dr. Matthew S. Goldberg (University of Texas Southwestern Medical Center, USA) for sharing a mouse Lrrk2 cDNA construct. This work was supported by the Intramural Research Program of the National Institute on Aging, NIH, by The Michael J. Fox Foundation for Parkinson’s Research, and by a Parkinson’s Foundation- American Parkinson Disease Association Summer Student Fellowship, PF-APDA-SFW-1742.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In honor of Prof. Anthony J. Turner.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2018_2650_MOESM1_ESM.pdf
Supplementary figure S1. Higher mouse LRRK2 protein expression is not explained by increased protein stability. A. HEK293FT cells were transfected with Flag-tagged human or mouse LRRK2 and subjected to a 35S-cysteine/35S-methionine “pulse” followed by a “chase” with media enriched with “cold” cysteine and methionine. Cell lysates were subjected to immunoprecipitation (IP) for Flag and exposed to a storage phosphor screen (upper panel), then blotted for Flag (lower panel). The representative blots shown are taken from an experiment in which cells were collected at 32 hours. In a separate experiment cells were collected at 2 hours, and not at 32 hours. B. Quantification of 35S-LRRK2 relative to Flag-LRRK2 for human and mouse LRRK2 from n=3 independent experiments, with technical n=3 for each construct at each time point. Error bars indicate SEM. The best-fit lines shown are semilog lines, where X is linear, and Y is log. A one phase decay non-linear regression equation was used to calculate half-life of each protein (Human LRRK2: half-life = 3.83h, 95%CI=2.71-5.68, R2=0.84. Mouse Lrrk2: half-life = 3.36h, 95%CI=2.60-4.38, R2=0.904). (PDF 252 KB)
11064_2018_2650_MOESM2_ESM.pdf
Supplementary figure S2. Mouse LRRK2 is expressed at higher levels than human protein in mouse cells. A-C. Primary mouse glial cells (A), N2a cells (B) or NIH 3t3 cells (C) were transfected with Flag-tagged human or mouse LRRK2, or mock transfected and protein levels measured by western blot using a flag antibody. Cyclophilin B is used as a loading control for each lane. (PDF 550 KB)
11064_2018_2650_MOESM3_ESM.pdf
Supplementary figure S3. Mouse and human LRRK2 self-interact at similar levels. A. HEK293FT cells were co-transfected with GFP-tagged LRRK2 and indicated Flag-tagged human or mouse LRRK2 constructs, with GUS as a negative control. Cell lysates were subjected to immunoprecipitation (IP) for GFP and blotted for GFP (upper panel) and Flag (lower panels). Inputs for the IP are shown on the left. B. Quantification of IP for Flag-tagged proteins relative to inputs for indicated human and mouse constructs from n=3 independent experiments. Error bars indicate SEM, **, p < 0.01; ns, non-significant by Tukey’s post-hoc test from one way ANOVA compared to WT human LRRK2. (PDF 104 KB)
11064_2018_2650_MOESM4_ESM.pdf
Supplementary figure S4. Enzyme activity of mouse and human LRRK2 estimated using model peptide, Nictide. Quantification of n=3 independent experiments using indicated constructs of the phosphorylation of the Nictide peptide. *, p < 0.05; ***, p < 0.001; ns, non-significant by Tukey’s post-hoc test compared to WT human LRRK2 from one way ANOVA (F10,55=20.87, p < 0.001, n=6 samples per construct). (PDF 58 KB)
Rights and permissions
About this article
Cite this article
Langston, R.G., Rudenko, I.N., Kumaran, R. et al. Differences in Stability, Activity and Mutation Effects Between Human and Mouse Leucine-Rich Repeat Kinase 2. Neurochem Res 44, 1446–1459 (2019). https://doi.org/10.1007/s11064-018-2650-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2650-4