Abstract
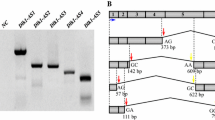
We cloned the cDNA and genomic DNA encoding for Izumo1 of cashmere goat (Capra hircus) and sheep (Ovis aries). Analysis of 4.6 kb Izumo1 genomic sequences in sheep and goat revealed a canonical open reading frame (ORF) of 963 bp spliced by eight exons. Sheep and goat Izumo1 genes share >99% identity at both DNA and protein levels and are also highly homologous to the orthologues in cattle, mouse, rat and human. Extensive cloning and analysis of Izumo1 cDNA revealed three (del 69, del 182 and del 217) and two (del 69 and ins 30) alternative splicing isoforms in goat and sheep, respectively. All of the isoforms are derived from splicing at typical GT-AG sites leading to partial or complete truncation of the immunoglobulin (Ig)-like domain. Bioinformatics analysis showed that caprine and ovine Izumo1 proteins share similar structure with their murine orthologue. There are a signal peptide at the N-terminus (1–22 aa), a transmembrane domain at the C-terminus (302–319 aa), and an extracellular Ig-like region in the middle (161–252 aa) with a putative N-linked glycosylation site (N205-N-S). Alignment of Izumo1 protein sequences among 15 mammalian species displayed several highly conserved regions, including LDC and YRC motifs with cysteine residues for potential disulfide bridge formation, CPNKCG motif upstream of the Ig-like domain, GLTDYSFYRVW motif upstream of the putative N-linked glycosylation site, and a number of scattered cysteine residues. These distinctive features are very informative to pinpoint the important gene motifs and functions. The C-terminal regions, however, are more variable across species. Izumo1 cDNA sequences of goat, sheep, and cow were found to be largely homologous, and the molecular phylogenetic analysis is consistent with their morphological taxonomy. This implies the Izumo1 gene evolves from the same ancestor, and the mechanism of sperm–egg fusion in mammals may be under the same principle in which Izumo1 plays an important role.






Similar content being viewed by others
References
Primakoff P, Myles DG (2000) The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet 16:83–87
Iba K, Albrechtsen R, Gilpin B, Fröhlich C, Loechel F, Zolkiewska A, Ishiguro K, Kojima T, Liu W, Langford JK, Sanderson RD, Brakebusch C, Fässler R, Wewer UM (2000) The cysteine-rich domain of humanADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading. J Cell Biol 149:1143–1156
McLaughlin EA, Frayne J, Bloomerg G, Hall L (2001) Do fertilin beta and cyritestin play a major role in mammalian sperm–oolemma interactions? A critical re-evaluation of the use of peptide mimics in identifying specific oocyte recognition proteins. Mol Hum Reprod 7:313–317
Yuan R, Primakoff P, Myles DG (1997) A role for the disintegrin domain of cyritestin, a sperm surface protein belonging to the ADAM family, in mouse sperm–egg plasma membrane adhesion and fusion. J Cell Biol 137:105–112
Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG (1998) Fertilization defects in sperm from mice lacking fertilin beta. Science 281:1857–1859
Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P (2001) Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev Biol 233:204–213
Shamsadin R, Adham IM, Nayernia K, Heinlein UA, Oberwinkler H, Engel W (1999) Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod 61:1445–1451
Cuasnicú PS, González Echeverría F, Piazza AD, Cameo MS, Blaquier JA (1984) Antibodies against epididymal glycoproteins block fertilizing ability in rat. J Reprod Fertil 72:467–471
Rochwerger L, Cohen DJ, Cuasnicú PS (1992) Mammalian sperm–egg fusion: the rat egg has complementary sites for a sperm protein that mediates gamete fusion. Dev Biol 153:83–90
Cohen DJ, Ellerman DA, Cuasnicú PS (2000) Mammalian sperm–egg fusion: evidence that epididymal protein DE plays a role in mouse gamete fusion. Biol Reprod 63:462–468
Ellerman DA, Brantúa VS, Martínez SP, Cohen DJ, Conesa D, Cuasnicú PS (1998) Potential contraceptive use of epididymal proteins: immunization of male rats with epididymal protein DE inhibits sperm fusion ability. Biol Reprod 59:1029–1036
Ellerman DA, Cohen DJ, Da Ros VG, Morgenfeld MM, Busso D, Cuasnicú PS (2006) Sperm protein ‘DE’ mediates gamete fusion through an evolutionarily conserved site of the CRISP family. Dev Biol 297:228–237
Stein KK, Go JC, Lane WS, Primakoff P, Myles DG (2006) Proteomic analysis of sperm regions that mediate sperm–egg interactions. Proteomics 6:3533–3543
Ellerman DA, Myles DG, Primakoff P (2006) A role for sperm surface protein disulfide isomerase activity in gamete fusion: evidence for the participation of ERp57. Dev Cell 10:831–837
Inoue N, Ikawa M, Isotani A, Okabe M (2005) The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434:234–238
Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C (2000) Severely reduced female fertility in CD9-deficient mice. Science 287:319–321
Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E (2000) Requirement of CD9 on the egg plasma membrane for fertilization. Science 287:321–324
Kaji K, Oda S, Miyazaki S, Kudo A (2002) Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm–egg fusion. Dev Biol 247:327–334
Rubinstein E, Ziyyat A, Wolf JP, Le Naour F, Boucheix C (2006) The molecular players of sperm–egg fusion in mammals. Semin Cell Dev Biol 17:254–263
Ziyyat A, Rubinstein E, Monier-Gavelle F, Barraud V, Kulski O, Prenant M, Boucheix C, Bomsel M, Wolf JP (2006) CD9 controls the formation of clusters that contain tetraspanins and the integrin alpha 6 beta 1, which are involved in human and mouse gamete fusion. J Cell Sci 119:416–424
Nixon B, Aitken RJ, McLaughlin EA (2007) New insights into the molecular mechanisms of sperm–egg interaction. Cell Mol Life Sci 64:1805–1823
Xing WJ, Wang LQ, Wu Q, Ren SC, Bao XH, Bou S (2009) Molecular cloning and characterization of CD9 cDNA from sheep and cashmere goat. Reprod Dom Anim [Epub ahead of print]
Schultz R, Williams C (2005) Developmental biology: sperm–egg fusion unscrambled. Nature 434:152–153
Toshimori K, Maekawa M, Ito C, Toyama Y, Suzuki-Toyota F, Saxena DK (2006) The involvement of immunoglobulin superfamily proteins in spermatogenesis and sperm–egg interaction. Reprod Med Biol 5:87–93
Saxena DK, Oh-Oka T, Kadomatsu K, Muramatsu T, Toshimori K (2002) Behaviour of a sperm surface transmembrane glycoprotein basigin during epididymal maturation and its role in fertilization in mice. Reproduction 123:435–444
Marshall RD (1972) Glycoproteins. Annu Rev Biochem 41:673–702
Chen W, Helenius J, Braakman I, Helenius A (1995) Cotranslational folding and calnexin binding during glycoprotein synthesis. Proc Natl Acad Sci USA 92:6229–6233
Meunier JC, Fournillier A, Choukhi A, Cahour A, Cocquerel L, Dubuisson J, Wychowski C (1999) Analysis of the glycosylation sites of the hepatitis C virus (HCV) glycoprotein E1 and the influence of E1 glycans on the formation of the HCV glycoprotein complex. J Gen Virol 80:887–896
Goffard A, Callens N, Bartosch B, Wychowski C, Cosset FL, Montpellier-Pala C, Dubuisson J (2005) Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J Virol 79:8400–8409
Akama TO, Nakagawa H, Sugihara K, Narisawa S, Ohyama C, Nishimura S, O’Brien DA, Moremen KW, Millan JL, Fukuda MN (2002) Germ cell survival through carbohydrate-mediated interaction with Sertoli cells. Science 295:124–127
Yamaguchi R, Yamagata K, Ikawa M, Moss SB, Okabe M (2006) Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice. Biol Reprod 75:760–766
Ikawa M, Nakanishi T, Yamada S, Wada I, Kominami K, Tanaka H, Nozaki M, Nishimune Y, Okabe M (2001) Calmegin is required for fertilin alpha/beta heterodimerization and sperm fertility. Dev Biol 240:254–261
Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, Nishimune Y, Okabe M (1997) The putative chaperone calmegin is required for sperm fertility. Nature 387:607–611
Ponce RH, Urch UA, Yanagimachi R (1994) Inhibition of sperm–egg fusion in the hamster and mouse by carbohydrates. Zygote 2:253–262
Inoue N, Ikawa M, Okabe M (2008) Putative sperm fusion protein IZUMO and the role of N-glycosylation. Biochem Biophys Res Commun 377:910–914
Javadpour MM, Eilers M, Groesbeek M, Smith SO (1999) Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys J 77:1609–1618
Cohen P (2000) The regulation of protein function by multisite phosphorylation—a 25 year update. Trends Biochem Sci 25:596–601
Acknowledgments
This work was funded by grants from Inner Mongolian Natural Science Foundation (20080404MS0505), The National Training Fund for Talents of Basic Sciences (J0730648) and The National High Technology Research and Development Program of China (2002AA242061).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xing, WJ., Han, BD., Wu, Q. et al. Molecular cloning and characterization of Izumo1 gene from sheep and cashmere goat reveal alternative splicing. Mol Biol Rep 38, 1995–2006 (2011). https://doi.org/10.1007/s11033-010-0322-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0322-9