Abstract
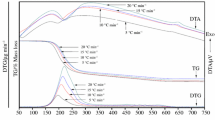
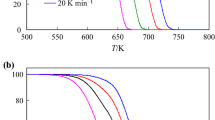
Kinetic triplet of the complex decomposition processes of Co3Ni3(PO4)2·8H2O was evaluated for the first time by using the deconvolution method to separate the overlapping DTG curves. After the completion of the deconvolution, five steps of the decomposition were obtained. The activation energy E and the pre-exponential factor A of each step were determined by KAS method. The kinetic compensation effect (KCE) method was applied to identify the individual step of the decomposition. Each master plot was simplified by generating the general equations and combined with the nonlinear regression curve fitting. According to kinetic analysis results obtained from this modified method, it was found that the early four steps of dehydration follow the mechanisms of nucleation and subsequent growth with different n-orders, while the last step occurs in the same mechanism but accompanied by the phase transition (lattice reorientation).














Similar content being viewed by others
References
Mesguer S, Tena MA, Gargori C, Galindo R, Badenes JA, Llusar M. Development of blue ceramic dyes from cobalt phosphates. Ceram Int. 2008;34(6):1431–8.
Mesguer S, Tena MA, Gargori C, Badenes JA, Llusar M, Monrόs G. Structure and colour of cobalt ceramic pigments from phosphates. Ceram Int. 2007;33(5):843–9.
Kullyakool S, Danvirutai C, Siriwong K, Noisong P. Thermal behavior, surface properties and vibrational spectroscopic studies of the synthesized Co3x Ni3−3x ·(PO4)2·8H2O (0 ≤ x ≤ 1). Solid State Sci. 2013;24:147–53.
Viter VN, Nagornyi PG. Synthesis and study of solid solutions between cobalt and nickel phosphates with varied degree of anion protonation. Zh Phrikl Khim. 2009;82(6):881–5.
Gralwey AK. Structure and order in thermal dehydration of crystalline solids. Thermochim Acta. 2000;355:181–238.
Gralwey AK. Is the science of thermal analysis kinetics based on solid foundations? A literature appraisal. Thermochim Acta. 2004;413:139–83.
Searcy AW, Berutot D. Kinetics of endothermic decomposition reactions. 2. Effects of the solid and gaseous products. J Phys Chem. 1978;82:2–6.
Boontima S, Danvirutai C, Srithanratana T. Thermal decomposition kinetics and reversible hydration study of the Li2Zn(HPO4)2·H2O. Solid State Sci. 2010;10:1226–30.
Kullyakool S, Siriwong K, Noisong P, Danvirutai C. Studies of thermal decomposition kinetics and temperature dependence of thermodynamic functions of the new precursor LiNiPO4·3H2O for the synthesis of olivine LiNiPO4. J Therm Anal Calorim. 2015;122:665–77.
Perejón A, Sáchez-Jiménez PE, Criado JM, Pérez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115:1780–91.
Cheng Z, Wu W, Ji P, Zhou X, Liu R, Cai J. Applicability of Fraser-Suzuki function in kinetic analysis of DAEM processes and lignocellulosic biomass pyrolysis processes. J Therm Anal Calorim. 2015;119:1429–38.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J. Res. Natl Bur Stand Sect A. 1966;70A(6):487–523.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep Chiba Inst Tech. 1964;20:22–3.
Vyazovkin S, Burnhan AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetic committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Xia Y, Huang Y, Li Y, Liao S, Long Q, Liang J. LiPO4: Ce, Tb, Yb Phosphor-synthesis and kinetics study for thermal process of precursor by Vyazovkin, OFW, KAS, Staring, and Mastplosts methods. J Therm Anal Calorim. 2015;120:1635–43.
Senum GI, Yang RT. Rational approximation of the integral of the Arrhenius function. J Therm Anal Calorim. 1977;11:445–7.
Gotor FJ, Criado MJ, Malek J, Koga N. Kinetic analysis of solid-state reaction: the universality of master plots for analyzing isothermal and nonisothermal experiments. J Phys Chem A. 2000;104:0777–10782.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of nonisothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrol. 2008;81(2):253–62.
Rooney JJ. Isokinetic temperature and the compensation effect in catalysis. J Mol Catal A Chem. 1998;133:303–5.
Karunakaran C, Chidambaranathan V. Linear free energy relationships near isokinetic temperature. Oxidation of organic sulfides with nicotinium dichromate. Croat Chem Acta. 2001;74(1):51–9.
Galwey AK, Brown ME. Arrhenius parameter and compensation behaviour in solid-state decompositions. Thermochim Acta. 1997;300:107–15.
Kullyakool S, Siriwong K, Noisong P, Danvirutai C. Determination of kinetic triplet of the synthesized Ni3(PO4)2·8H2O by non-isothermal and isothermal kinetic methods. J Therm Anal Calorim. 2014;115:1497–507.
Khawam A, Flanagan DR. Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B. 2006;110:17315–28.
Koga N, Šimon P, Šesták J. Some fundamental and historical aspects of phenomenological kinetics in solid-state studied by thermal analysis, chap. 1. In: Šesták J, Šimon P, editors. Thermal analysis of micro-, nano- and non-crystalline materials. Berlin: Springer; 2013. p. 1–28.
Acknowledgements
We thank the Department of Chemistry and the Department of Physics (for XRD), Faculty of Science, Khon Kaen University, for providing research facilities. The financial support from the Center for Innovation in Chemistry (PERCH-CIC), Ministry of Education as well as from the Higher Education Research Promotion and National Research University Project of Thailand, Office of Higher Education Commission, through the Advanced Functional Materials Cluster of Khon Kaen University (contract number NRU544018), is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kullyakool, S., Siriwong, K., Noisong, P. et al. Kinetic triplet evaluation of a complicated dehydration of Co3(PO4)2·8H2O using the deconvolution and the simplified master plots combined with nonlinear regression. J Therm Anal Calorim 127, 1963–1974 (2017). https://doi.org/10.1007/s10973-016-5837-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5837-4