Abstract
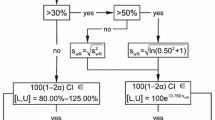
Regulatory authorities introduced procedures in the last decade for evaluating the bioequivalence (BE) for highly variable drugs. These approaches are similar in principle but differ in details. For example, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) recommend differing regulatory constants. The constant suggested by FDA results in discontinuity of the BE limits around the switching variation at 30% observed within-subject variation of the reference product. The regulatory constant of EMA does not have these problems. The Type I error reaches 6–7% around the switching variation with the EMA constant but 16–17% with the FDA constant. Various procedures were recently suggested, especially for the EMA approach, to eliminate the inflation of the Type I error. Notably, the so-called Exact algorithms try to amalgamate the positive features of both EMA and FDA procedures without their negative sides. The computational procedure for the EMA approach is simple and has a straightforward interpretation. The procedure for the FDA approach is based on an approximation, has a bias at small degrees of freedom, and requires a suitable computer program. All regulatory agencies impose a second requirement constraining the point estimate of the ratio of geometric means. In addition, EMA and Health Canada impose an upper limit for applying the recommended procedures. These expectations have psychological motivation and political rationale but no scientific foundations. Their inclusion results in incorrect and misleading interpretation of the principal criterion which involves confidence intervals. Different regulatory authorities expect to apply their approaches either to both AUC and Cmax or only to AUC or only to Cmax. Rational resolution of the disharmonization is needed.


Similar content being viewed by others
References
Shah VP, Yacobi A, Barr WH, Benet LZ, Breimer D, Dobrinska MR, Endrenyi L et al (1996) Evaluation of orally administered highly variable drugs and drug formulations. Pharm Res 13:1590–1594
Davit BM, Conner DP, Fabian-Fritsch B, Haidar SH, Jiang X, Patel DT et al (2008) Highly variable drugs: observations from bioequivalence data submitted to the FDA for New Generic Drug Applications. AAPS J 10:148–156
Karalis V, Macheras P, Van Peer A, Shah VP (2008) Bioavailability and bioequivalence: focus on physiological factors and variability. Pharm Res 25:1956–1963
Cook CS (2011) Current issues on bioavailability and bioequivalence determination. J Bioeq Bioavail S1–003:1–5
Marzo A, Vuksic D, Crivelli F (2000) Bioequivalence of endogenous substances facing homeostatic equilibria: an example with potassium. Pharmacol Res 42:523–525
Ehmann F, Sakai-Kato K, Duncan R, de la Ossa DHP, Pita R, Vidal JM, Kohli A, Tothfalusi L et al (2013) Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomed 8:849–856
Schuirmann DJ (1987) A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 15:657–680
Schall R (1995) A unified view of individual, population, and average bioequivalence. In: Blume H, Midha K (eds) Bio-International 2, bioavailability, bioequivalence and pharmacokinetics. Medpharm, Stuttgart, pp 91–106
Tothfalusi L, Endrenyi L, Midha K, Rawson MJ, Hubbard JW (2001) Evaluation of the bioequivalence of highly-variable drugs and drug products. Pharm Res 18:728–733
Haidar SH, Davit B, Chen ML, Conner D, Lee L, Li QH, Lionberger R, Makhlouf F, Patel D, Schuirmann DJ, Yu LX (2008) Bioequivalence approaches for highly variable drugs and drug products. Pharm Res 25:237–241
Tothfalusi L, Endrenyi L, Garcia Areta A (2009) Evaluation of bioequivalence for highly variable drugs with scaled average bioequivalence. Clin Pharmacokinet 48:725–743
Dragalin V, Fedorov V, Patterson S, Jones B (2003) Kullback–Leibler divergence for evaluating bioequivalence. Stat Med 22:913–930
Schall R, Williams RL (1996) Towards a practical strategy for assessing individual bioequivalence. J Pharmacokinet Biopharm 24:133–149
Patnaik RN, Lesko LJ, Chen ML, Williams RL (1997) Individual bioequivalence: new concenpts in the statististical assessment of bioequivalence metrics. Clin Pharmacokinet 33:1–6
Davit BM, Chen ML, Conner DP, Haidar SH, Kim S, Lee CH, Lionberger RA, Makhlouf FT, Nwakama PE, Patel DT, Schuirmann DJ, Yu LX (2012) Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products by the US Food and Drug Administration. AAPS J 14:915–924
Food and Drug Administration (2013) Draft guidance for industry: Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA. Center for Drug Evaluation and Research (CDER), Silver Spring, MD https://www.fda.gov/downloads/drugs/guidances/ucm377465.pdf
Davit BM, Conner DP (2017) The United States. In: Kanfer I (ed) Bioequivalence requirements in various global jurisdictions. Springer, Cham, pp 269–305
Food and Drug Administration (2011) Draft guidance for industry: Bioequivalence recommendations for progesterone oral capsules. Center for Drug Evaluation and Research (CDER), Silver Spring, MD https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM209294.pdf
Hyslop T, Hsuan F, Holder DJ (2000) A small sample confidence interval approach to assess individual bioequivalence. Stat Med 19:2885–2897
Tothfalusi L, Endrenyi L (2003) Limits for the scaled average bioequivalence of highly variable drugs and drug products. Pharm Res 20:382–389
European Medicines Agency (2010) Guideline on the investigation of bioequivalence. London, United Kingdom http://www.ema.europa.eu/docs/en_GB/document_library/2010/01/WC500070039.pdf
Boddy AW, Snikeris FC, Kringle RO, Wei GC, Oppermann JA, Midha KK (1995) An approach for widening the bioequivalence acceptance limits in the case of highly variable drugs. Pharm Res 12:1865–1868
European Medicines Agency (2015) Questions & Answers: positions on specific questions addressed to the Pharmacokinetics Working Party (PKWP). 19 November 2015 EMA/618604/2008 Rev. 13 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002963.pdf. Accessed 7 Aug 2018
Haidar SH, Makhlouf F, Schuirmann DJ, Hyslop T, Davit B, Conner D, Yu LX (2008) Evaluation of a scaling approach for the bioequivalence of highly variable drugs. AAPS J 10:450–454
Endrenyi L, Tothfalusi L (2009) Regulatory conditions for the determination of bioequivalence of highly variable drugs. J Pharm Pharmaceut Sci 12:138–149
Labes D (2013) ‘Alpha’ of scaled ABE. Bioequivalence and bioavailability forum. BEBAC Consultancy Services for Bioequivalence and Bioavailability Studies, Vienna http://forum.bebac.at/mix_entry.php?id=10202
Wonnemann M, Frőmke C, Koch A (2015) Inflation of the type I error: investigations on regulatory recommendations for bioequivalence of highly variable drugs. Pharm Res 32:135–143
Munoz J, Alcaide D, Ocana J (2016) Consumer’s risk in the EMA and FDA regulatory approaches for bioequivalence in highly variable drugs. Stat Med 35:1933–1943
Labes D, Schütz H (2016) Inflation of type I error in the evaluation of scaled average bioequivalence, and a method for its control. Pharm Res 33:2805–2814
Tothfalusi L, Endrenyi L (2016) An exact procedure for the evaluation of reference scaled average bioequivalence. AAPS J 18:476–489
Tothfalusi L, Endrenyi L (2017) Algorithms for evaluating reference scaled average bioequivalence: power, bias, and consumer risk. Stat Med 36:4378–4390
Patterson SD, Jones B (2012) Viewpoint: observations on scaled average bioequivalence. Pharm Stat 11:1–7
Hedges LV (1981) Distribution theory for Glass’s estimator of effect size and related estimator. J Educ Stat 6:107–128
Benet L (2006) Why highly variable drugs are safer. Meeting of FDA Committee for Pharmaceutical Science. http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4241s2_2.htm. Accessed 6 Oct 2006
Karalis V, Symillides M, Macheras P (2004) Novel scaled average bioequivalence limits based on GMR and variability considerations. Pharm Res 21:1933–1942
Karalis V, Macheras P, Symillides M (2005) Geometric mean ratio-dependent scaledbioequivalence limits with leveling-off properties. Eur J Pharm Sci 26:54–61
Kytariolos J, Karalis V, Macheras P, Symillides M (2006) Novel scaled bioequivalence limits with leveling-off properties. Pharm Res 23:2657–2664
Karalis V, Symillides M, Macheras P (2011) On the leveling-off properties of the new bioequivalence limits for highly variable drugs of the EMA guideline. Eur J Pharm Sci 44:497–505
Acknowledgement
We appreciate the very careful and thoughtful review of the original manuscript by two referees.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Endrenyi, L., Tothfalusi, L. Bioequivalence for highly variable drugs: regulatory agreements, disagreements, and harmonization. J Pharmacokinet Pharmacodyn 46, 117–126 (2019). https://doi.org/10.1007/s10928-019-09623-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-019-09623-w