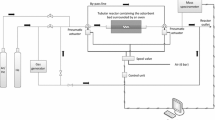
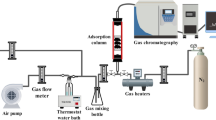
Abstract
The effect of the micropore structure of activated carbon fibers (ACFs) on the adsorption of toluene at low concentration (10–80 ppmv) was studied over two types of ACFs. Both adsorbents presented similar surface chemistry but different porous structure: one ACF was ultramicroporous (d pore < 1 nm) and the other supermicroporous (d pore ~1 to 2 nm). Toluene adsorption isotherms were determined for both ACFs and were found to be consistent with Dubinin–Radushkevich (D–R) model. The toluene adsorption enthalpies calculated from temperature-programmed desorption profiles at temperatures below 330 K were close to the values obtained from D–R equations. Larger adsorption strength was found in the ultramicroporous adsorbent as compared to the supermicroporous one suggesting that the pore shape strongly influences the adsorption mechanism. The adsorbent with a lower specific surface area but narrower micropores could be more efficient at high temperatures than an adsorbent with large pore volume and wider micropores. The findings reported have a big importance for correct choice of material when designing efficient structured adsorbing bed.









Similar content being viewed by others
References
Baur, G.B., Beswick, O., Spring, J., Yuranov, I., Kiwi-Minsker, L.: Activated carbon fibers for efficient VOC removal from diluted streams: the role of surface functionalities. Adsorption (2015a). doi:10.1007/s10450-015-9667-7
Baur, G.B., Yuranov, I., Kiwi-Minsker, L.: Activated carbon fibers modified by metal oxide as effective structured adsorbents for acetaldehyde. Catal. Today (2015b). doi:10.1016/j.cattod.2014.11.021
Cal, M.P., Rood, M.J., Larson, S.M.: Gas phase adsorption of volatile organic compounds and water vapor on activated carbon cloth. Energy Fuels 11(2), 311–315 (1997)
Cazorla-Amoros, D., Alcaniz-Monge, J., de la Casa-Lillo, M.A., Linares-Solano, A.: CO2 as an adsorptive to characterize carbon molecular sieves and activated carbons. Langmuir 14(16), 4589–4596 (1998)
Cazorla-Amoros, D., Alcaniz-Monge, J., Linares-Solano, A.: Characterization of activated carbon fibers by CO2 adsorption. Langmuir 12(11), 2820–2824 (1996)
Cvetanović, R.J., Amenomiya, Y.: Application of a temperature-programmed desorption technique to catalyst studies. In: Eley, D.D., Pines, H., Paul, B.W. (eds.) Advances in Catalysis, vol. 17, pp. 103–149. Academic Press, New York (1967)
Das, D., Gaur, V., Verma, N.: Removal of volatile organic compound by activated carbon fiber. Carbon 42(14), 2949–2962 (2004)
Dimotakis, E.D., Cal, M.P., Economy, J., Rood, M.J., Larson, S.M.: Chemically treated activated carbon cloths for removal of volatile organic carbons from gas streams—evidence for enhanced physical adsorption. Environ. Sci. Technol. 29(7), 1876–1880 (1995)
Dubinin, M.M.: The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 60(2), 235–241 (1960)
Dubinin, M.M.: Adsorption in micropores. J. Colloid Interface Sci. 23(4), 487–499 (1967)
Dubinin, M.M.: Fundamentals of the theory of adsorption in micropores of carbon adsorbents—characteristics of their adsorption properties and microporous structures. Carbon 27(3), 457–467 (1989)
Dubinin, M.M., Stoeckli, H.F.: Homogeneous and heterogeneous micropore structures in carbonaceous adsorbents. J. Colloid Interface Sci. 75(1), 34–42 (1980)
Duong, D.D.: Adsorption Analysis: Equilibria and Kinetics. Series on chemical engineering, vol. 2. Imperial College Press, London (1998)
El-Sayed, Y., Bandosz, T.J.: A study of acetaldehyde adsorption on activated carbons. J. Colloid Interface Sci. 242(1), 44–51 (2001)
Figueiredo, J.L., Pereira, M.F.R., Freitas, M.M.A., Orfao, J.J.M.: Modification of the surface chemistry of activated carbons. Carbon 37(9), 1379–1389 (1999)
Foster, K.L., Fuerman, R.G., Economy, J., Larson, S.M., Rood, M.J.: Adsorption characteristics of trace volatile organic-compounds in gas streams onto activated carbon-fibers. Chem. Mater. 4(5), 1068–1073 (1992)
Garrido, J., Linaressolano, A., Martinmartinez, J.M., Molinasabio, M., Rodriguezreinoso, F., Torregrosa, R.: Use of N2 vs Co2 in the characterization of activated carbons. Langmuir 3(1), 76–81 (1987)
Hayashi, T., Kumita, M., Otani, Y.: Removal of acetaldehyde vapor with impregnated activated carbons: effects of steric structure on impregnant and acidity. Environ. Sci. Technol. 39(14), 5436–5441 (2005)
Hutson, N.D., Yang, R.T.: Theoretical basis for the Dubinin–Radushkevitch (D–R) adsorption isotherm equation. Adsorpt. J. Int. Adsorpt. Soc. 3(3), 189–195 (1997)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
Lillo-Rodenas, M.A., Cazorla-Amoros, D., Linares-Solano, A.: Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 43(8), 1758–1767 (2005)
Lillo-Rodenas, M.A., Cazorla-Amoros, D., Linares-Solano, A.: Benzene and toluene adsorption at low concentration on activated carbon fibres. Adsorpt. J. Int. Adsorpt. Soc. 17(3), 473–481 (2011)
Lillo-Rodenas, M.A., Fletcher, A.J., Thomas, K.M., Cazorla-Amoros, D., Linares-Solano, A.: Competitive adsorption of a benzene–toluene mixture on activated carbons at low concentration. Carbon 44(8), 1455–1463 (2006)
Mangun, C.L., Benak, K.R., Economy, J., Foster, K.L.: Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 39(12), 1809–1820 (2001)
Mangun, C.L., Daley, M.A., Braatz, R.D., Economy, J.: Effect of pore size on adsorption of hydrocarbons in phenolic-based activated carbon fibers. Carbon 36(1–2), 123–129 (1998)
Piccot, S.D., Watson, J.J., Jones, J.W.: A global inventory of volatile organic-compound emissions from anthropogenic sources. J. Geophys. Res. Atmos. 97(D9), 9897–9912 (1992)
Pitzer, K.S., Scott, D.W.: The thermodynamics and molecular structure of benzene and its methyl derivatives. J. Am. Chem. Soc. 65, 803–829 (1943)
Popescu, M., Joly, J.P., Carre, J., Danatoiu, C.: Dynamical adsorption and temperature-programmed desorption of VOCs (toluene, butyl acetate and butanol) on activated carbons. Carbon 41(4), 739–748 (2003)
Singh, K.P., Mohan, D., Tandon, G.S., Gupta, G.S.D.: Vapor-phase adsorption of hexane and benzene on activated carbon fabric cloth: equilibria and rate studies. Ind. Eng. Chem. Res. 41(10), 2480–2486 (2002)
Tancrede, M., Wilson, R., Zeise, L., Crouch, E.A.C.: The carcinogenic risk of some organic vapors indoors—a theoretical survey. Atmos. Environ. 21(10), 2187–2205 (1987)
Terzyk, A.P., Gauden, P.A., Kowalczyk, P.: What kind of pore size distribution is assumed in the Dubinin–Astakhov adsorption isotherm equation? Carbon 40(15), 2879–2886 (2002)
Wu, J.F., Stromqvist, M.E., Claesson, O., Fangmark, I.E., Hammarstrom, L.G.: A systematic approach for modelling the affinity coefficient in the Dubinin–Radushkevich equation. Carbon 40(14), 2587–2596 (2002)
Acknowledgments
Research described in this article was supported by Philip Morris International.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baur, G.B., Yuranov, I., Renken, A. et al. Activated carbon fibers for efficient VOC removal from diluted streams: the role of surface morphology. Adsorption 21, 479–488 (2015). https://doi.org/10.1007/s10450-015-9685-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9685-5