Abstract
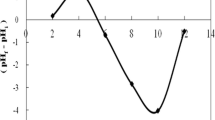
Adsorption of vanadium(V) from aqueous solution onto ZnCl2 activated carbon developed from coconut coir pith was investigated to assess the possible use of this adsorbent. The influence of various parameters such as agitation time, vanadium concentration, adsorbent dose, pH and temperature has been studied. First, second order, Elovich and Bangham’s models were used to study the adsorption kinetics. The adsorption system follows second order and Bangham’s kinetic models. Langmuir, Freundlich, Dubinin-Radushkevich and Temkin isotherms have been employed to analyze the adsorption equilibrium data. Equilibrium adsorption data followed all the four isotherms—Langmuir, Freundlich, D-R and Temkin. The Langmuir adsorption capacity (Q 0) was found to be 24.9 mg g− 1 of the adsorbent. The per cent adsorption was maximum in the pH range 4.0–9.0. The pH effect and desorption studies showed that ion exchange mechanism might be involved in the adsorption process. Thermodynamic parameters such as ΔG 0, ΔH 0 and ΔS 0 for the adsorption were evaluated. Effect of competitive anions in the aqueous solution such as PO4 3 −, SO4 2−, ClO4 −, MoO4 2−, SeO3 2−, NO3 − and Cl− was examined. SEM and FTIR were used to study the surface of vanadium(V) loaded ZnCl2 activated carbon. Removal of vanadium(V) from synthetic ground water was also tested. Results show that ZnCl2 activated coir pith carbon is effective for the removal of vanadium(V) from water.
Similar content being viewed by others
References
Abbas, A. and B.E. Conway, “Investigation of Removal of Cr(VI), MO(VI), W(VI), V(IV) and V(V) Oxyions from Industrial Wastewaters by Adsorption and Electrosorption at High-area Carbon Cloth,” J. Colloid Interf. Sci., 251, 248–55 (2002).
Alkan, M., O. Demirbas, S. Celikcapa and M. Dogan, “Sorption of Acid Red 57 from Aqueous Solution Anto Sepiolite,” J. Hazard Mater., B116, 135–145 (2004).
Annual Book of ASTM Standards, Section 15, “General Products,” in Chemical Specialities and End Use Products—Activated Carbon, ASTM International, West Conshohocken, PA, USA, Vol. 15.01 (1999).
Aoki, T. and M. Munemori, “Recovery of Cr(VI) from Wastewaters with Iron(III) Hydroxide, I. Adsorption Mechanism of Chromium(VI) on Iron(III) Hydroxide,” Water Res., 16, 793–796 (1982).
APHA, Standard Methods for the Examination of Water and Wastewater, 18th edn. American Public Health Association, Washington, DC pp. 330–331 (1992).
Bellamy, L.J., The Infrared Spectra of Complex Molecules, 3rd edn. Chapman and Hall, London, Vol. 1, p. 368 (1975).
Bhargava, D.S. and S.B. Sheldarkar, “Use of TNSAC in Phosphate Adsorption Studies and Relationships, Literature, Experimental Methodology, Justification and Effects of Process Variables,” Water Res., 27, 303–12 (1993).
Bhatnagar, A. and A.K. Jain, “A Comparative Adsorption Study with Different Industrial Wastes as Adsorbents for the Removal of Cationic Dyes from Water,” J. Colloid Interf. Sci., 281, 49–55 (2005).
Blackmore, D.P.T., J. Ellis, and P.J. Riley, “Treatment of a Vanadium-Containing Effluent by Adsorption/Co precipitation with Iron Oxyhydroxide,” Water Res., 30, 2512–2516 (1996).
Bureau of Indian Standards, Discharge Limits of the Effluents, IS 2490: 1981, New Delhi, 1981.
Cater, D.E. and F. Quintus, “Chemical Toxicology, Part II. Metal Toxicity,” J. Chem. Educ., 56, 490–495 (1979).
Cheung, C.W., J.F. Porter, and G. McKay, “Sorption Kinetics for the Removal of Copper and Zinc from Effluents using Bone char”, Sep. Purif. Technol., 19, 55–64 (2000).
Choy, K.K.H., G. McKay and J.F. Porter, “Sorption of Acid Dyes from Effluents Using Activated Carbon,” Resour. Conserv. Recy., 27, 57–71 (1999).
Freundlich, H., “Uber Die Adsorption in Losungen,” Z. Phys. Chem., 57, 387–470 (1906).
Gopal, M. and R.A. Gupta, “Coir Waste for a Scientific Cause,” Indian Coconut J., 31,13–16 (2001).
Guzman, J., I. Saucedo, R. Navarro, J. Revilla, and E. Guibal, “Vanadium Interactions with Chitosan: Influence of Polymer Protonation and Metal Speciation,” Langmuir, 18, 1567–1573 (2002).
Hall, K.R., L.C. Eagleton, A. Acrivos, and T. Vermeulen, “Pore and Solid Diffusion Kinetics Infixed Bed Adsorption under Constant Pattern Conditions,” Ind. Eng. Chem. Fund., 5, 212–223 (1966).
Ho, Y.S. and G. McKay, “Sorption of Dye from Aqueous Solution by Peat,” Chem. Eng. J., 70, 115–124 (1998).
Jansson-Charrier, M., E. Guibal, J. Roussy, B. Delanghe, and P. Le cloirec, “Vanadium(IV) Sorption by Chitosan: Kinetics and Equilibrium,” Water Res., 30, 465–475 (1996).
Jiang, Z., Y. Liu, X. Sun, F. Tian, F. Sun, C. Liang, W. You, C. Han, and C. Li, “Activated Carbons Chemically Modified by Concentrated H2SO4 for the Adsorption of the Pollutants from Wastewater and the Dibenzothiphene from Fuel Oils. Langmuir, 19, 731–736 (2003).
Kabata-Pendias, A. and H. Pendias, Biochemistry of Trace Elements, PWN, Warsaw, Poland, 1993.
Kabata-Pendias, A. and H. Pendias, Rare Elements in Biological Environment, Geological Publication, Warsaw, Poland, 1979.
Kunz, R.G., J.F. Giannelli, and H.D. Stensel, “Vanadium Removal from Industrial Wastewaters,” J. Water Pollut. Con. F., 48, 762–70 (1976).
Lagergren, S., “Zur Theorie Der Sogenannten Adsorption Gel- oester Stoffe,” Kungliga Svenska Vetenskapsakadomiens Handlinger., 24, 1–39 (1898).
Laidler, K.J. and J.H. Meiser, Physical Chemistry, Houghton Mittin, New York, 1999.
Langmuir, I., “The Adsorption of Gases on Plane Surface of Glass, Mica and Platinum,” J. Am. Chem. Soc., 40, 1361 (1918).
Laszlo, K. and A. Szucs, “Surface Characterization of Polyethyleneterephthalate (PET) based Activated Carbon and the Effect of pH on its Adsorption Capacity from Aqueous Phenol and 2,3,4-trichlorophenol Solutions”, Carbon, 39, 1945–1953 (2001).
Mahmut, O., “Equilibrium and Kinetic Modeling of Adsorption of Phosphorous on Calcined Alunite,” Adsorption, 9, 125–132 (2003).
Namasivayam, C. and D. Kavitha, “Adsorptive Removal of 2-Chlorophenol by Low-Cost Coir Pith Carbon,” J. Hazard. Mater., 98, 257–274 (2003).
Namasivayam, C. and D. Sangeetha, “Equilibrium and Kinetic Studies of Adsorption of Phosphate onto ZnCl2 Activated Coir Pith Carbon,” J. Colloid Interf. Sci., 280, 359–365 (2004).
Ozcan, A.S., B. Erdem, and A. Ozcan, “Adsorption of Acid blue 913 from Aqueous Solutions onto BTMA-bentonite”, Colloid Surface A, 266, 73–81 (2005).
Partitt, R.L., “Anion Adsorption by Soils and Soil Materials,” Advances in Agronomy, 30, 1–50 (1978).
Prange, A. and K. Kremling, “Distribution of Dissolved Molybdenum, Uranium and Vanadium in Baltic Sea Waters,” Mar. Chem., 16, 259–274 (1985).
Sabio, M., and F.R. Reinoso, “Role of Chemical Activation in the Development and Carbon Porosity,” Colloid Surfaces A, 241, 15–25 (2004).
Smisek, M., and S. Cerney, Activated Carbon, Manufacture, Properties and Applications, Elsevier, New York, pp. 10–32 1970.
Sparks, D.L., “Kinetics of Sorption/Release Reactions at the Soil Mineral/Water Interface,” In D.L. Sparks (ed.), Soil Physical Chemistry, 2nd edition, CRC Press, Boca Raton, FL, pp. 135–191, (1999).
Vega, E.D., J.C. Pedregosa, G.E. Narda, and P.J. Morando, “Removal of Oxovanadium(IV) from Aqueous Solutions by Using Commercial Crystalline Calcium Hydroxyapatite,” Water. Res., 37, 1776–1782 (2003).
Vinodhini, S., V.S. Gnanambal, and S.N. Padmadevi, “Efficacy of Degraded Coir Pith for the Growth of Medicinal Plants,” Indian Coconut. J. 35, 16-1-7 (2005).
Wu, F.C., R.L. Tseng, and R.S. Juang, “Preparation of Highly Microporous Carbons From Fir Wood by KOH Activation for Adsorption of Dyes and Phenols from Water,” Sep. Purif. Technol. 47, 10–19 (2005).
Zagulski, I., L. Pawlowski, and A. Cichocki, “Physicochemical Methods for Water and Wastewater Treatment,” In L. Pawlowski L, (Ed.), Proceedings of the Second International Conference, Lublin 1979. Pergamon Press, Oxford, UK, (1980).
Zeng, L., X. Li, and J. Liu, “Adsorptive Removal of Phosphate from Aqueous Solutions Using Iron Oxide Tailings,” Water Res., 38, 1318–1326 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Namasivayam, C., Sangeetha, D. Removal and recovery of vanadium(V) by adsorption onto ZnCl2 activated carbon: Kinetics and isotherms. Adsorption 12, 103–117 (2006). https://doi.org/10.1007/s10450-006-0373-3
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10450-006-0373-3