Abstract
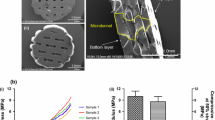
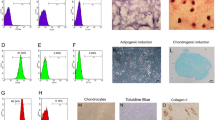
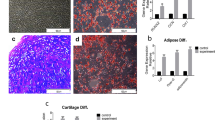
The main objective of this study was to evaluate the effectiveness of a mesenchymal stem cell (MSC)-seeded polyethylene-oxide-terephthalate/polybutylene-terephthalate (PEOT/PBT) scaffold for cartilage tissue repair in an osteochondral defect using a rabbit model. Material characterisation using scanning electron microscopy indicated that the scaffold had a 3D architecture characteristic of the additive manufacturing fabrication method, with a strut diameter of 296 ± 52 μm and a pore size of 512 ± 22 μm × 476 ± 25 μm × 180 ± 30 μm. In vitro optimisation revealed that the scaffold did not generate an adverse cell response, optimal cell loading conditions were achieved using 50 μg/ml fibronectin and a cell seeding density of 25 × 106 cells/ml and glycosaminoglycan (GAG) accumulation after 28 days culture in the presence of TGFβ3 indicated positive chondrogenesis. Cell-seeded scaffolds were implanted in osteochondral defects for 12 weeks, with cell-free scaffolds and empty defects employed as controls. On examination of toluidine blue staining for chondrogenesis and GAG accumulation, both the empty defect and the cell-seeded scaffold appeared to promote repair. However, the empty defect and the cell-free scaffold stained positive for collagen type I or fibrocartilage, while the cell-seeded scaffold stained positive for collagen type II indicative of hyaline cartilage and was statistically better than the cell-free scaffold in the blinded histological evaluation. In summary, MSCs in combination with a 3D PEOT/PBT scaffold created a reparative environment for cartilage repair.






Similar content being viewed by others
References
Anderson, J. A., D. Little, A. P. Toth, C. T. Moorman, B. S. Tucker, M. G. Ciccotti, and F. Guilak. Stem cell therapies for knee cartilage repair: the current status of preclinical and clinical studies. Am. J. Sports Med. 2013. doi:10.1177/0363546513508744.
Bhosale, A. M., and J. B. Richardson. Articular cartilage: structure, injuries and review of management. Br. Med. Bull. 87:77–95, 2008.
Christensen, B. B., C. B. Foldager, O. M. Hansen, A. A. Kristiansen, D. Q. S. Le, A. D. Nielsen, J. V. Nygaard, C. E. Bünger, and M. Lind. A novel nano-structured porous polycaprolactone scaffold improves hyaline cartilage repair in a rabbit model compared to a collagen type I/III scaffold: in vitro and in vivo studies. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 20:1192–1204, 2012.
Chu, C. R., M. Szczodry, and S. Bruno. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 16:105–115, 2010.
Emans, P. J., E. J. P. Jansen, D. van Iersel, T. J. M. Welting, T. B. F. Woodfield, S. K. Bulstra, J. Riesle, L. W. van Rhijn, and R. Kuijer. Tissue-engineered constructs: the effect of scaffold architecture in osteochondral repair. J. Tissue Eng. Regen. Med., 2012. doi:10.1002/term.1477.
Hoemann, C., R. Kandel, S. Roberts, D. B. F. Saris, L. Creemers, P. Mainil-Varlet, S. Méthot, A. P. Hollander, and M. D. Buschmann. International cartilage repair society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage 2:153–172, 2011.
Hurtig, M. B., M. D. Buschmann, L. A. Fortier, C. D. Hoemann, E. B. Hunziker, J. S. Jurvelin, P. Mainil-Varlet, C. W. McIlwraith, R. L. Sah, and R. A. Whiteside. Preclinical studies for cartilage repair recommendations from the international cartilage repair society. Cartilage 2:137–152, 2011.
Jansen, E. J. P., J. Pieper, M. J. J. Gijbels, N. A. Guldemond, J. Riesle, L. W. Van Rhijn, S. K. Bulstra, and R. Kuijer. PEOT/PBT based scaffolds with low mechanical properties improve cartilage repair tissue formation in osteochondral defects. J. Biomed. Mater. Res. A 89:444–452, 2009.
Johnson, K., S. Zhu, M. S. Tremblay, J. N. Payette, J. Wang, L. C. Bouchez, S. Meeusen, A. Althage, C. Y. Cho, X. Wu, and P. G. Schultz. A stem cell-based approach to cartilage repair. Science 336:717–721, 2012.
Kavalkovich, K. W., R. E. Boynton, J. M. Murphy, and F. Barry. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. Vitro Cell. Dev. Biol. Anim. 38:457–466, 2002.
Le Saux, G., A. Magenau, T. Böcking, K. Gaus, and J. J. Gooding. The relative importance of topography and RGD ligand density for endothelial cell adhesion. PLoS ONE 6:e21869, 2011.
Lee, C. H., J. L. Cook, A. Mendelson, E. K. Moioli, H. Yao, and J. J. Mao. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376:440–448, 2010.
Liu, Y., X. Z. Shu, and G. D. Prestwich. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 12:3405–3416, 2006.
Longo, U. G., S. Petrillo, E. Franceschetti, A. Berton, N. Maffulli, and V. Denaro. Stem cells and gene therapy for cartilage repair. Stem Cells Int. 2012:168385, 2012.
Mackle, J. N., D. J.-P. Blond, E. Mooney, C. McDonnell, W. J. Blau, G. Shaw, F. P. Barry, J. M. Murphy, and V. Barron. In vitro characterization of an electroactive carbon-nanotube-based nanofiber scaffold for tissue engineering. Macromol. Biosci. 11:1272–1282, 2011.
Maehara, H., S. Sotome, T. Yoshii, I. Torigoe, Y. Kawasaki, Y. Sugata, M. Yuasa, M. Hirano, N. Mochizuki, M. Kikuchi, K. Shinomiya, and A. Okawa. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 28:677–686, 2010.
Maheshwari, G., G. Brown, D. A. Lauffenburger, A. Wells, and L. G. Griffith. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113:1677–1686, 2000.
Malda, J., T. B. F. Woodfield, F. van der Vloodt, C. Wilson, D. E. Martens, J. Tramper, C. A. van Blitterswijk, and J. Riesle. The effect of PEGT/PBT scaffold architecture on the composition of tissue engineered cartilage. Biomaterials 26:63–72, 2005.
Massia, S. P., and J. A. Hubbell. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 114:1089–1100, 1991.
Matsiko, A., T. J. Levingstone, F. J. O’Brien, and J. P. Gleeson. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 11:41–52, 2012.
Moroni, L., J. R. de Wijn, and C. A. van Blitterswijk. Three-dimensional fiber-deposited PEOT/PBT copolymer scaffolds for tissue engineering: influence of porosity, molecular network mesh size, and swelling in aqueous media on dynamic mechanical properties. J. Biomed. Mater. Res. A 75:957–965, 2005.
Moroni, L., G. Poort, F. Van Keulen, J. R. de Wijn, and C. A. van Blitterswijk. Dynamic mechanical properties of 3D fiber-deposited PEOT/PBT scaffolds: an experimental and numerical analysis. J. Biomed. Mater. Res. A 78:605–614, 2006.
Moutos, F. T., B. T. Estes, and F. Guilak. Multifunctional hybrid three-dimensionally woven scaffolds for cartilage tissue engineering. Macromol. Biosci. 10:1355–1364, 2010.
Moutos, F. T., L. E. Freed, and F. Guilak. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 6:162–167, 2007.
Murphy, J. M., D. J. Fink, E. B. Hunziker, and F. P. Barry. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48:3464–3474, 2003.
Reinholz, G. G., L. Lu, D. B. F. Saris, M. J. Yaszemski, and S. W. O’Driscoll. Animal models for cartilage reconstruction. Biomaterials 25:1511–1521, 2004.
Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697–715, 1996.
Shao, X., J. C. H. Goh, D. W. Hutmacher, E. H. Lee, and G. Zigang. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 12:1539–1551, 2006.
Shapiro, F., S. Koide, and M. J. Glimcher. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 75:532–553, 1993.
Solchaga, L. A., J. U. Yoo, M. Lundberg, J. E. Dennis, B. A. Huibregtse, V. M. Goldberg, and A. I. Caplan. Hyaluronan-based polymers in the treatment of osteochondral defects. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 18:773–780, 2000.
Valonen, P. K., F. T. Moutos, A. Kusanagi, M. G. Moretti, B. O. Diekman, J. F. Welter, A. I. Caplan, F. Guilak, and L. E. Freed. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials 31:2193–2200, 2010.
Woodfield, T. B. F., J. Malda, J. de Wijn, F. Péters, J. Riesle, and C. A. van Blitterswijk. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 25:4149–4161, 2004.
Acknowledgments
This work was supported by Science Foundation Ireland (SFI) Strategic Research Cluster (SRC), Grant No. SFI: 09/SRC B1794, Wellcome Trust Biomedical Vacation Scholarships Grant Number WTD004448, the European Union’s 7th Framework Programme under Grant Agreement No. HEALTH-2007-B-223298 (PurStem).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kent Leach oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Barron, V., Merghani, K., Shaw, G. et al. Evaluation of Cartilage Repair by Mesenchymal Stem Cells Seeded on a PEOT/PBT Scaffold in an Osteochondral Defect. Ann Biomed Eng 43, 2069–2082 (2015). https://doi.org/10.1007/s10439-015-1246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1246-2