Abstract
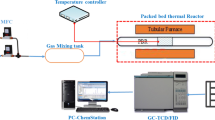
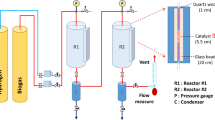
This study focuses on the potential of hydrogen-rich syngas production by CO2 reforming of methane over Co/Pr2O3 catalyst. The Co/Pr2O3 catalyst was synthesized via wet-impregnation method and characterized for physicochemical properties by TGA, XRD, BET, H2-TPR, FESEM, EDX, and FTIR. The CO2 reforming of methane over the as-synthesized catalyst was studied in a tubular stainless steel fixed-bed reactor at feed ratio ranged 0.1–1.0, temperature ranged 923–1023 K, and gas hourly space velocity (GHSV) of 30,000 h−1 under atmospheric pressure condition. The catalyst activity studies showed that the increase in the reaction temperature from 923 to 1023 K and feed ratio from 0.1 to 1.0 resulted in a corresponding increase in the reactant’s conversion and the product’s yields. At 1023 K and feed ratio of 1.0, the activity of the Co/Pr2O3 catalyst climaxed with CH4 and CO2 conversions of 41.49 and 42.36 %. Moreover, the catalyst activity at 1023 K and feed ratio of 1.0 resulted in the production of H2 and CO yields of 40.7 and 40.90 %, respectively. The syngas produced was estimated to have H2:CO ratio of 0.995, making it suitable as chemical building blocks for the production of oxygenated fuel and other value-added chemicals. The used Co/Pr2O3 catalyst which was characterized by TPO, XRD, and SEM-EDX show some evidence of carbon formation and deposition on its surface.












Similar content being viewed by others
References
Abasaeed AE, Al-fatesh AS, Naeem MA, Ibrahim AA, Fakeeha AH (2015) Catalytic performance of CeO2 and ZrO2 supported Co catalysts for hydrogen production via dry reforming of methane. Int Hydrog Energy 40:6818–6826. doi:10.1016/j.ijhydene.2015.03.152
Alifanti M, Bueno G, Parvulescu V, Parvulescu VI, Cortés Corberán V (2009) Oxidation of ethane on high specific surface SmCoO3 and PrCoO3 perovskites. Catal Today 143:309–314. doi:10.1016/j.cattod.2009.02.026
Ayodele BV, Cheng CK (2015) Modelling and optimization of syngas production from methane dry reforming over ceria-supported cobalt catalyst using artificial neural networks and Box-Behnken design. J Ind Eng Chem. doi:10.1016/j.jiec.2015.08.021
Ayodele BV, Khan MR, Cheng CK (2015a) Syngas production from CO2 reforming of methane over ceria supported cobalt catalyst: effects of reactants partial pressure. J Nat Gas Sci Eng. doi:10.1016/j.jngse.2015.09.049
Ayodele BV, Khan MR, Cheng CK (2015b) Catalytic performance of ceria-supported cobalt catalyst for CO-rich hydrogen production from dry reforming of methane. Int J Hydrog Energy 41:198–207. doi:10.1016/j.ijhydene.2015.10.049
Ayodele BV, Hossain MA, Chong SL, Soh JC, Abdullah S, Khan MR, Cheng CK (2016a) Non-isothermal kinetics and mechanistic study of thermal decomposition of light rare earth metal nitrate hydrates using thermogravimetric analysis. J Therm Anal Calorim. doi:10.1007/s10973-016-5450-6
Ayodele BV, Khan MR, Lam SS, Cheng CK (2016b) Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: kinetic and mechanistic studies. J Hydrog Energy, Int. doi:10.1016/j.ijhydene.2016.01.091
Balboul BAA (2010) Synthesis course and surface properties of praseodymium oxide obtained via thermal decomposition of praseodymium acetate: impacts of the decomposition atmosphere. J Anal Appl Pyrolysis 88:192–198. doi:10.1016/j.jaap.2010.04.006
Baliban RC, Elia JA, Weekman V, Floudas CA (2012) Process synthesis of hybrid coal, biomass, and natural gas to liquids via Fischer–Tropsch synthesis, ZSM-5 catalytic conversion, methanol synthesis, methanol-to-gasoline, and methanol-to-olefins/distillate technologies. Comput Chem Eng 47:29–56. doi:10.1016/j.compchemeng.2012.06.032
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J Am Chem Soc 73:373–380. doi:10.1021/ja01145a126
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. doi:10.1021/ja01269a023
Burton AW, Ong K, Rea T, Chan IY (2009) On the estimation of average crystallite size of zeolites from the Scherrer equation: a critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater 117:75–90. doi:10.1016/j.micromeso.2008.06.010
Chaubey R, Sahu S, James OO, Maity S (2013) A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew Sustain Energy Rev 23:443–462. doi:10.1016/j.rser.2013.02.019
Cipriani G, Di Dio V, Genduso F, La Cascia D, Liga R, Miceli R, Ricco Galluzzo G (2014) Perspective on hydrogen energy carrier and its automotive applications. Int J Hydrog Energy 39:8482–8494. doi:10.1016/j.ijhydene.2014.03.174
Donohue M, Aranovich G (1998) Classification of Gibbs adsorption isotherms. Adv Colloid Interface Sci 76–77:137–152. doi:10.1016/S0001-8686(98)00044-X
Ehrhardt C, Gjikaj M, Brockner W (2005) Thermal decomposition of cobalt nitrato compounds: preparation of anhydrous cobalt(II)nitrate and its characterisation by infrared and Raman spectra. Thermochim Acta 432:36–40. doi:10.1016/j.tca.2005.04.010
Ferencz Zs, Baán K, Oszkó A, Kónya Z, Kecskés T, Erdőhelyi A (2014) Dry reforming of CH4 on Rh doped Co/Al2O3 catalysts. Catal Today 228:123–130. doi:10.1016/j.cattod.2013.11.014
Gahleitner G (2013) Hydrogen from renewable electricity: an international review of power-to-gas pilot plants for stationary applications. Int J Hydrog Energy 38:2039–2061. doi:10.1016/j.ijhydene.2012.12.010
Garavaglia R, Mari CM, Trasatti S (1984) Physicochemical characterization of Co3O4 prepared by thermal decomposition II: response to solution pH. Surf Technol 23:41–47. doi:10.1016/0376-4583(84)90074-8
Haag S, Burgard M, Ernst B (2007) Beneficial effects of the use of a nickel membrane reactor for the dry reforming of methane: comparison with thermodynamic predictions. J Catal 252:190–204. doi:10.1016/j.jcat.2007.09.022
Hafizi A, Rahimpour MR, Hassanajili S (2016) Hydrogen production by chemical looping steam reforming of methane over Mg promoted iron oxygen carrier: optimization using design of experiments. J Taiwan Inst Chem Eng. doi:10.1016/j.jtice.2016.01.023
Han C, Wu J, Pu C, Qiao S, Wu B, Zhu J, Xiao D (2012) High piezoelectric coefficient of Pr2O3-doped Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics. Ceram Int 38:6359–6363. doi:10.1016/j.ceramint.2012.05.008
Hussein GA, Balboul BA, A-Warith M, Othman AG (2001) Thermal genesis course and characterization of praseodymium oxide from praseodymium nitrate hydrate. Thermochim Acta 369:59–66. doi:10.1016/S0040-6031(00)00727-9
Itkulova SS, Zhunusova KZ, Zakumbaeva GD (2005) CO2 reforming of methane over Co-Pd/Al2O3 catalysts. Bull Korean Chem Soc 26(12):2017–2020. doi:10.5012/bkcs.2005.26.12.2017
Ji YG, Zhao Z, Duan A, Jiang GY, Liu J (2009) Comparative study on the formation and reduction of bulk and Al2O3-supported cobalt oxides by H-2-TPR technique. J Phys Chem B 113:7186–7199. doi:10.1021/jp8107057
Konnov AA, De Ruyck J (2001) Temperature-dependent rate constant for the reaction NNH + O → NH + NO. Combust Flame 125:1258–1264
Li Y, Tan B, Wu Y (2008) Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett 8:265–270. doi:10.1021/nl0725906
Luisetto I, Tuti S, Di Bartolomeo E (2012) Co and Ni supported on CeO2 as selective bimetallic catalyst for dry reforming of methane. Int J Hydrog Energy 37:15992–15999. doi:10.1016/j.ijhydene.2012.08.006
Muraza O, Galadima A (2015) A review on coke management during dry reforming of methane. Int J Energy Res 39:1196–1216
Nagaoka K (2003) Influence of the reduction temperature on catalytic activity of Co/TiO2 (anatase-type) for high pressure dry reforming of methane. Appl Catal A Gen 255:13–21. doi:10.1016/S0926-860X(03)00631-8
Ruckenstein E, Wang H (2000) Carbon dioxide reforming of methane to synthesis gas over supported cobalt catalysts. Appl Catal A Gen 204:257–263. doi:10.1016/S0926-860X(00)00674-8
Sehested J (2006) Four challenges for nickel steam-reforming catalysts. Catal Today 111:103–110. doi:10.1016/j.cattod.2005.10.002
Sharaf OZ, Orhan MF (2014) An overview of fuel cell technology: fundamentals and applications. Renew Sustain Energy Rev 32:810–853. doi:10.1016/j.rser.2014.01.012
Shrestha S, Yeung CMY, Nunnerley C, Tsang SC (2007) Comparison of morphology and electrical conductivity of various thin films containing nano-crystalline praseodymium oxide particles. Sens Actuators A Phys 136:191–198. doi:10.1016/j.sna.2006.11.019
Silva JM, Soria MA, Madeira LM (2015) Challenges and strategies for optimization of glycerol steam reforming process. Renew Sustain Energy Rev 42:1187–1213. doi:10.1016/j.rser.2014.10.084
Tang C-W, Wang C-B, Chien S-H (2008) Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim Acta 473:68–73. doi:10.1016/j.tca.2008.04.015
Tsoukalou A, Imtiaz Q, Kim SM, Abdala PM, Yoon S, Müller CR (2016) Dry-reforming of methane over bimetallic Ni–M/La2O3 (M=Co, Fe): the effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J Catal. doi:10.1016/j.jcat.2016.03.018
Wang N, Chu W, Huang L, Zhang T (2010) Effects of Ce/Zr ratio on the structure and performances of Co–Ce1−xZrxO2 catalysts for carbon dioxide reforming of methane. J Nat Gas Chem 19:117–122. doi:10.1016/S1003-9953(09)60055-4
Wilhelm D, Simbeck D, Karp A, Dickenson R (2001) Syngas production for gas-to-liquids applications: technologies, issues and outlook. Fuel Process Technol 71:139–148. doi:10.1016/S0378-3820(01)00140-0
Xiong H, Moyo M, Motchelaho MA, Tetana ZN, Dube SM, Jewell LL, Coville NJ (2014) Fischer–Tropsch synthesis: iron catalysts supported on N-doped carbon spheres prepared by chemical vapor deposition and hydrothermal approaches. J Catal 311:80–87. doi:10.1016/j.jcat.2013.11.007
Acknowledgments
The authors would like to acknowledge the research fund RDU130501 granted by the Ministry of Science, Technology and Innovation Malaysia (MOSTI). Bamidele Victor Ayodele gratefully appreciates the Universiti Malaysia Pahang for the provision of Doctoral Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayodele, B.V., Khan, M.R. & Cheng, C.K. Greenhouse gases mitigation by CO2 reforming of methane to hydrogen-rich syngas using praseodymium oxide supported cobalt catalyst. Clean Techn Environ Policy 19, 795–807 (2017). https://doi.org/10.1007/s10098-016-1267-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1267-z