Abstract
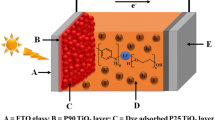
Polymer gel electrolytes based on poly(acrylic acid)-poly(ethylene glycol) (PAA–PEG) hybrid have been prepared and applied to developed quasi-solid-state dye-sensitized solar cells (DSCs). PAA–PEG hybrid was synthesized by polymerization reaction. Quasi-solid-state DSCs were fabricated with synthesized PAA–PEG electrolyte. The effects of alkali iodides LiI, KI, and I2 concentrations on liquid electrolyte absorbency and ionic conductivity of PAA–PEG were investigated. The evolution of the solar cell parameters with polymer gel electrolyte compositions was revealed. DSCs based on PAA–PEG with optimized KI/I2 concentrations showed better performances than those with optimized LiI/I2 concentrations. The electrochemical impedance spectroscopy technique was employed to examine the electron lifetime in the TiO2 electrode and quantify charge transfer resistances at the TiO2/dye/electrolyte interface and the counter electrode in the solar cells based on the PAA–PEG hybrid gels. A maximum conversion efficiency of 4.96% was obtained for DSCs using KI based quasi-solid electrolyte under 100 mW cm−2. Our work suggests that KI can be the promising alkali metal iodide for improving the performance of PAA–PEG hybrid gel DSCs.





Similar content being viewed by others
References
O’Regan B, Grätzel M (1991) Nature 353:737–740
Tennakone K, Perera V, Kottegoda I, Kumara G (1999) J Phys D Appl Phys 32:374–379
Bach U, Lupo D, Comte P, Moser JE (1998) Nature 395:583–585
Wang P, Zakeeruddin SM, Moser JE, Nazeeruddin MK, Sekiguchi T, Grätzel M (2003) Nat Mater 2:402–407
Stergiopoulos T, Arabatzis IM, Katsaros G, Falaras P (2002) Nano Lett 2:1259–1261
Katsaros G, Stergiopoulos T, Arabatzis IM, Papadokostaki KG, Falaras P (2002) J Photochem Photobiol A 149:191–198
Kim YJ, Kim JH, Kang MS, Lee MJ, Won J, Lee JC, Kang YS (2004) Adv Mater 16:1753–1757
Cao F, Oskam G, Searson PC (1995) J Phys Chem 99:17071–17080
Yang H, Huang ML, Wu JH, Lan Z, Hao SC, Lin JM (2008) Mater Chem Phys 110:38–42
Zhang J, Han HW, Wu SJ, Xu S, Zhou CH, Yang Y, Zhao XZ (2007) Nanotechnology 18:295606
Zhang DW, Li XD, Sun Z, Chen YW, Huang SM (2008) The 2nd IEEE International Nanoelectronics Conference (INEC 2008):1335–1339
Lan Z, Wu JH, Lin JM, Huang ML (2007) J Power Sources 164:921–925
Lan Z, Wu JH, Lin JM, Huang ML, Li PJ, Li QH (2008) Electrochim Acta 53:2296–2301
Wu JH, Lan Z, Lin JM, Huang ML, Hao SC, Sato T, Yin S (2007) Adv Mater 19:4006–4011
Ito S, Chen P, Comte P (2007) Prog Photovolt 15:603–612
Ito S, Nazeeruddin M, Liska P, Comte P (2006) Prog Photovolt 14:589–601
Kim JH, Kang MS, Kim YJ, Won J, Kang YS (2005) Solid State Ionics 176:579–584
Liu Y, Hagfeldt A, Xiao XR, Lindquist SE (1998) Sol Energy Mater Sol Cells 55:267–281
Peter LM (2007) Phy Chem Chem Phys 9:2630–2642
Hara K, Horiguchi T, Kinoshita T, Sayama K, Arakawa H (2001) Sol Energy Mater Sol Cells 70:151–161
Kern R, Sastrawan R, Ferber J, Stangl R, Luther J (2002) Electrochim Acta 47:4213–4225
Wang Q, Moser JE, Grätzel M (2005) J Phys Chem B 109:14945–14953
Han L, Koide N, Chiba Y, Islam A, Komiya R, Fuke N, Fukui A, Yamanaka RR (2005) Appl Phys Lett 86:213501–213504
Zhang DW, Li XD, Chen S, Tao F, Sun Z, Yin XJ, Huang SM (2009) J Solid State Electrochem. doi:10.1007/s10008-009-0982-3
Kusama H, Kurashige M, Arakawa H (2005) J Photochem Photobiol A: Chem 169:169–176
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 10774046), Shanghai Municipal Science and Technology Committee (No.09JC1404600, No.0852 nm06100 and No.08230705400) and Singapore Ministry of Education innovation fund (MOE IF Funding MOE2008-IF-1-016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Zhang, D., Yin, X.J. et al. The effects of polymer gel electrolyte composition on performance of quasi-solid-state dye-sensitized solar cells. J Solid State Electrochem 15, 1271–1277 (2011). https://doi.org/10.1007/s10008-010-1201-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1201-y