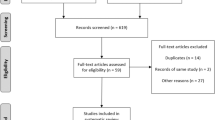
Abstract
A number of studies have assessed the efficacy of alglucosidase alfa as an enzyme replacement therapy (ERT) on motor and respiratory endpoints in patients with late-onset Pompe disease (LOPD). A previous review evaluated the clinical efficacy and safety of alglucosidase alfa; however, it is difficult to draw inferences from individual studies due to small patient populations, particularly in evaluating the benefit on survival. To evaluate the current evidence on the long-term efficacy of alglucosidase alfa with regard to survival, motor, and respiratory function in patients with LOPD in relation to the natural progression of the disease, a new systematic literature review was performed identifying studies that assessed either mortality, percent predicted forced vital capacity (% FVC), or the 6-min walk test (6MWT) among treated and untreated LOPD patients. Patient overlap was avoided by removing smaller studies or ensuring the use of only one conflicting study per outcome. Mortality was modeled using Poisson models for each treatment group. Outcomes were modeled using first- and second-order fractional polynomial meta-analysis with fixed- and random-effects. Meta-regression was used to explore sources of heterogeneity. Twenty-two publications pertaining to 19 studies/trials were selected, including 438 patients when accounting for overlaps, with the average study duration being 45.7 months. Patients treated with alglucosidase alfa in these studies had a nearly five-fold lower mortality rate than untreated patients (rate ratio: 0.21; 95 % credible interval: 0.11, 0.41). On average, % FVC declined consistently among untreated patients, including a 2.3 % decline after 12 months follow-up and 6.2 % decline after 48 months. This is in contrast to alglucosidase alfa-treated patients, who, on average, improved rapidly, with an increase of 1.4 % FVC after 2 months, followed by a slow regression back to baseline over a three-year period. Nonetheless, the relative difference between those treated and not grew over time, from 4.5 % FVC after 12 months to 6 % FVC after 48 months. In the 6MWT, alglucosidase alfa-treated patients on average had the largest improvement over the first 20 months of treatment of approximately 50 meters increase over baseline, with its substantial stabilization in the following years. By comparison, untreated patients do not show 6MWT improvement over time. Alglucosidase alfa has a beneficial effect in LOPD patients as demonstrated by improvements in survival and ambulation maintained over time, as well as prevention of deterioration in respiratory function.




Similar content being viewed by others
References
Ausems MG, Verbiest J, Hermans MP et al (1999) Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. Eur J Hum Genet 7(6):713–716
Hirschhorn R, Reuser A (2001) Glycogen storage disease Type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver C, Beaudet A, Sly W, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 3389–3420
Lashley FR (2005) Clinical genetics in nursing practice, 3rd edn. Springer Publishing Company Inc., New York
Meikle PJ, Hopwood JJ, Clague AE, Carey WF (1999) Prevalence of lysosomal storage disorders. JAMA 281(3):249–254
Hagemans ML, Winkel LP, Hop WC, Reuser AJ, Van Doorn PA, Van der Ploeg AT (2005) Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology 64(12):2139–2141
Cupler EJ, Berger KI, Leshner RT et al (2012) Consensus treatment recommendations for late onsent Pompe disease. Muscle Nerve 45(3):319–333
van der Ploeg AT, Clemens PR, Corzo D et al (2010) A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med 362(15):1396–1406
van der Ploeg AT, Barohn R, Carlson L et al (2012) Open-label extension study following the late-onset treatment study (LOTS) of alglucosidase alfa. Mol Genet Metab 107(3):456–461
Wokke JH, Escolar DM, Pestronk A et al (2008) Clinical features of late-onset pompe disease: a prospective cohort study. Muscle Nerve 38(4):1236–1245
Toscano A, Schoser B (2013) Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J Neurol 260(4):951–959
Anderson L, Henley W, Wyat K et al (2014) Effectiveness of enzyme replacement therapy in adults with late-onset Pompe disease: results from the NCS-LSD cohort study. J Inherit Metab Dis 37:945–952
Quanjer P, Tammeling G, Cotes J, Pedersen O, Peslin R, Yernault J (1993) Lung volumes and forced ventilatory flows. Eur Respir J 6(Suppl 16):5–40
Dias S, Sutton AJ, Welton NJ, Ades AE (2013) Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making 33(5):618–640
Dempster AP (1997) The direct use of likelihood for significance testing. Statist Comput 7(4):247–252
Spiegelhalter DJ, Abrams KR, Myles JP (2004) Bayesian approaches to clinical trials and health-care evaluation. Wiley, Chichester
Gungor D, Kruijshaar M, Plug I et al. (2013) Impact of enzyme replacement therapy on survival in adults with Pompe disease: results from a prospective international observational study. Orphanet J Rare Dis 8:49
Van Der Beek NA, De Vries JM, Hagemans ML et al. (2012) Clinical features and predictors for disease natural progression in adults with Pompe disease: a nationwide prospective observational study. Orphanet J Rare Dis 7:88
Van der Beek NA, Hagemans ML, Reuser AJ et al (2009) Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul Disord 19(2):113–117
Andreassen C, Schlutter J, Vissing J, Andersen H (2014) Effect of enzyme replacement therapy on isokinetic strength for all major muscle groups in four patients with Pompe disease-a long-term follow-up. Mol Genet Metab 112(1):40–43
Angelini C, Semplicini C, Ravaglia S et al (2012) New motor outcome function measures in evaluation of Late-Onset Pompe disease before and after enzyme replacement therapy. Muscle Nerve 45(6):831–834
Bembi B, Pisa FE, Confalonieri M et al (2010) Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J Inherit Metab Dis 33(6):727–735
Orlikowski D, Pellegrini N, Prigent H et al (2011) Recombinant human acid alpha-glucosidase (rhGAA) in adult patients with severe respiratory failure due to Pompe disease. Neuromuscul Disord 21(7):477–482
De Vries JM, Van Der Beek NA, Hop WC et al. (2012) Effect of enzyme therapy and prognostic factors in 69 adults with Pompe disease: an open-label single-center study. Orphanet J Rare Dis 7:73
Furusawa Y, Mori-Yoshimura M, Yamamoto T et al (2012) Effects of enzyme replacement therapy on five patients with advanced late-onset glycogen storage disease type II: a 2-year follow-up study. J Inherit Metab Dis 35(2):301–310
Merk T, Wibmer T, Schumann C, Kruger S (2009) Glycogen storage disease type II (Pompe disease)—influence of enzyme replacement therapy in adults. Eur J Neurol 16(2):274–277
Papadimas GK, Spengos K, Konstantinopoulou A et al (2011) Adult Pompe disease: clinical manifestations and outcome of the first Greek patients receiving enzyme replacement therapy. Clin Neurol Neurosurg 113(4):303–307
Patel TT, Banugaria SG, Case LE, Wenninger S, Schoser B, Kishnani PS (2012) The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol Genet Metab 106(3):301–309
Restel M, Bochynska A, Chahwan M et al (2014) Encymatic replacement therapy in patients with late-onset Pompe’s disease—a 5-year follow up. Eur J Neurol 21:527
Strothotte S, Strigl-Pill N, Grunert B et al (2010) Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J Neurol 257(1):91–97
van Capelle CI, van der Beek NA, Hagemans ML et al (2010) Effect of enzyme therapy in juvenile patients with Pompe disease: a three-year open-label study. Neuromuscul Disord 20(12):775–782
van Capelle CI, Winkel LP, Hagemans ML et al (2008) Eight years experience with enzyme replacement therapy in two children and one adult with Pompe disease. Neuromuscul Disord 18(6):447–452
Hartung R, Chamsi-Bacha F, Beck M, Mengel E (2007) Initial therapy response of 6 months of enzyme replacement therapy in 11 Juvenile/Adult M. Pompe patients. Clin Ther 29(Suppl C):S86–S87
Schneider I, Hanisch F, Muller T, Schmidt B, Zierz S (2013) Respiratory function in late-onset Pompe disease patients receiving long-term enzyme replacement therapy for more than 48 months. Wien Med Wochenschr 163(1–2):40–44
Regnery C, Kornblum C, Hanisch F et al (2012) 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J Inherit Metab Dis 35(5):837–845
Angelini C, Semplicini C, Tonin P et al (2009) Progress in enzyme replacement therapy in glycogen storage disease type II. Ther Adv Neurol Disord 2(3):143–153
Vianello A, Semplicini C, Paladini L et al (2013) Enzyme replacement therapy improves respiratory outcomes in patients with late-onset type II glycogenosis and high ventilator dependency. Lung 191(5):537–544
Ravaglia S, Danesino C, Moglia A et al (2010) Changes in nutritional status and body composition during enzyme replacement therapy in adult-onset type II glycogenosis. Eur J Neurol 17(7):957–962
Angelini C, Semplicini C, Ravaglia S et al (2012) Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J Neurol 259(5):952–958
Hagemans MLC, Hop WJC, Van Doom PA, Reuser AJJ, Van Der Ploeg AT (2006) Course of disability and respiratory function in untreated late-onset Pompe disease. Neurology 66(4):581–583
Gungor D, De Vries J, Brusse E et al. (2013) Enzyme replacement therapy and fatigue in adults with Pompe disease. BMC Musculoskelet Disord 109(2):174–178
Lachmann R, Schoser B (2013) The clinical relevance of outcomes used in late-onset Pompe disease: can we do better? Orphanet J Rare Dis 8:160
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BS has received research support, honoraria, and travel funding from Sanofi Genzyme during the past 5 years. Dr. Schoser is member of the Genzyme Pompe Disease Global Advisory Board. Dr. Schoser received honoraria and travel funding as member of the Global Advisory Boards from Biomarin Pharmaceutical, Amicus Therapeutics, and Audentes Therapeutics. AS is an employee of Sanofi Genzyme and stockholder of Sanofi. SK is an employee of Redwood Outcomes, a research consulting firm owned by Precision for Medicine and, holds no Sanofi stock. AH is an employee of Sanofi Genzyme and stockholder of Sanofi. JJ is an employee of Redwood Outcomes, a research consulting firm owned by Precision for Medicine and, holds no Sanofi stock. KC is an employee of Redwood Outcomes, a research consulting firm owned by Precision for Medicine, and holds no Sanofi stock. MK was an employee of Redwood Outcomes, a research consulting firm owned by Precision for Medicine, and holds no Sanofi stock. In the last 5 years, AT has received, from Sanofi Genzyme, travel grants and honoraria for teaching courses, lectures and, being also a member of the Genzyme Pompe Disease Global Advisory Board for his participation to the related meetings. Prof. Toscano has also received travel grants and honoraria from Biomarin Pharmaceuticals for his participation to the Biomarin Pompe Advisory Board meetings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schoser, B., Stewart, A., Kanters, S. et al. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis. J Neurol 264, 621–630 (2017). https://doi.org/10.1007/s00415-016-8219-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8219-8