Abstract
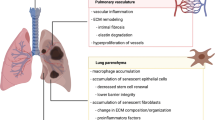
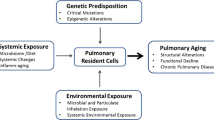
The incidence of chronic respiratory diseases (e.g., chronic obstructive pulmonary disease, COPD) and interstitial lung diseases (e.g., pneumonia and lung fibrosis) increases with age. In addition to immune senescence, the accumulation of senescent cells directly in lung tissue might play a critical role in the increased prevalence of these pulmonary diseases. In the last couple of years, detailed studies have identified the presence of senescent cells in the aging lung and in diseased lungs of patients with COPD and lung fibrosis. Cellular senescence has been shown for epithelial cells of bronchi and alveoli as well as mesenchymal and vascular cells. Known risk factors for pulmonary diseases (cigarette smoke, air pollutions, bacterial infections, etc.) were identified in experimental studies as being possible mediators in the development of cellular senescence. The present findings indicate the importance of cellular senescence in normal lung aging and in premature aging of the lung in patients with COPD, lung fibrosis, and probably other respiratory diseases.
Zusammenfassung
Chronische Atemwegserkrankungen, insbesondere die chronisch obstruktive Lungenerkrankung (COPD), sowie interstitielle Lungenerkrankungen wie die Pneumonie und die Lungenfibrose nehmen mit dem Alter deutlich zu. Dabei könnte neben der Immunseneszenz auch das vermehrte Auftreten von seneszenten Zellen direkt in der Lunge eine wichtige Rolle spielen. In den letzten Jahren wiesen detaillierte Studien seneszente Zellen in der alten Lunge und in der erkrankten Lunge von Patienten mit COPD oder Lungenfibrose nach. Neben den epithelialen Zellen von Bronchien und Alveolen waren von der Seneszenz auch Bindegewebszellen und Gefäßzellen betroffen. Mithilfe experimenteller Studien wurden bekannte Risikofaktoren von Atemwegserkrankungen (Zigarettenrauch, Luftverschmutzung, bakterielle Infektionen etc.) als mögliche Auslöser der zellulären Seneszenz identifiziert. Die Erkenntnisse weisen auf die Bedeutung der zellulären Seneszenz bei der normalen Lungenalterung hin, aber auch bei der verfrühten Alterung der Lunge bei Patienten mit COPD, Lungenfibrose oder möglicherweise anderen Atemwegserkrankungen.



Similar content being viewed by others
Abbreviations
- 53BP1:
-
p53-binding protein-1
- ATM:
-
ataxia telangiectasia-mutated
- ATR:
-
ATM- and Rad-3-related kinase
- CDC:
-
cell division cycle
- CDK:
-
cyclin-dependent kinase
- Chk:
-
checkpoint kinase
- COPD:
-
chronic obstructive pulmonary disease
- CKI:
-
cyclin-dependent kinase inhibitor
- DDR:
-
DNA damage response
- DNA-PK:
-
DNA-dependent protein kinase
- ds:
-
double stand
- GADD45:
-
growth arrest and DNA damage 45
- HR:
-
homologue recombination
- hTERT:
-
telomerase catalytic subunit
- IPF:
-
idiopathic pulmonary fibrosis
- ICD-10:
-
international statistical classification of diseases and related health problems, version 10
- IL:
-
interleukin
- MAPK:
-
mitogen-activated protein kinase
- MCP-1:
-
monocyte chemotactic protein-1
- mTOR:
-
mammalian target of rapamycin
- NAD:
-
nicotinamide adenine dinucleotide
- NHEJ:
-
non-homologue end-joining
- NF-κB:
-
nuclear factor κ-light-chain-enhancer of activated B-cells
- P:
-
phosphorylated
- p16Ink4a :
-
protein 16 (CKI 2A)
- p19Arf :
-
protein 19 (CKI 2A)
- p21Cip1/Waf1 :
-
protein 21 (cyclin-dependent kinase inhibitor protein 1A)
- p53:
-
protein 53
- pRb:
-
retinoblastoma susceptibility protein
- SA:
-
senescence-associated
- SAHF:
-
senescence-associated heterochromatin foci
- SAM:
-
senescence accelerated mouse
- SIRT:
-
sirtuin (silent mating type information regulation)
- Src:
-
Swiss raid commando
References
Taylor NAS (2011) Pulmonary function in aging humans. In: Masoro EJ, Austad, SN (eds) Handbook of the biology of aging. Elsevier, Academic Press, pp 421–446
Kerstjens HA, Rijcken B, Schouten JP et al (1997) Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 52:820–827
Sint T, Donohue JF, Ghio AJ (2008) Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease. Inhal Toxicol 20:25–29
Milara J, Cortijo J (2012) Tobacco, inflammation, and respiratory tract cancer. Curr Pharm Des 18:3901–3938
Statistisches Bundesamt, Zweigstelle Bonn (eds) (2011) Sterbefälle ab 1998. Gesundheitsberichterstattung des Bundes. http://www.gbe-bund.de. Accessed: 12 August 2013
Decramer M, Janssens W, Miravitlles M (2012) Chronic obstructive pulmonary disease. Lancet 379:1341–1351
Meyer KC (2010) The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly. Semin Respir Crit Care Med 31:561–574
Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88:557–579
Morla M, Busquets X, Pons J et al (2006) Telomere shortening in smokers with and without COPD. Eur Respir J 27:525–528
Lee J, Sandford AJ, Connett JE et al (2012) The relationship between telomere length and mortality in chronic obstructive pulmonary disease (COPD). PLoS One 7:e35567
Baur JA, Zou Y, Shay JW et al (2001) Telomere position effect in human cells. Science 292:2075–2077
Mallette FA, Ferbeyre G (2007) The DNA damage signaling pathway connects oncogenic stress to cellular senescence. Cell Cycle 6:1831–1836
Toussaint O, Remacle J, Dierick JF et al (2002) From the Hayflick mosaic to the mosaics of ageing. Role of stress-induced premature senescence in human ageing. Int J Biochem Cell Biol 34:1415–1429
Gorbunova V, Seluanov A (2005) Making ends meet in old age: DSB repair and aging. Mech Ageing Dev 126:621–628
Zglinicki T von, Saretzki G, Ladhoff J et al (2005) Human cell senescence as a DNA damage response. Mech Ageing Dev 126:111–117
Yang J, Yu Y, Hamrick HE et al (2003) ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis 24:1571–1580
Houtgraaf JH, Versmissen J, Giessen WJ van der (2006) A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med 7:165–172
Lin AW, Barradas M, Stone JC et al (1998) Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev 12:3008–3019
Sanchez-Perez Y, Chirino YI, Osornio-Vargas AR et al (2009) DNA damage response of A549 cells treated with particulate matter (PM10) of urban air pollutants. Cancer Lett 278:192–200
Alder JK, Guo N, Kembou F et al (2011) Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med 184:904–912
Muller M, Li Z, Maitz PK (2009) Pseudomonas pyocyanin inhibits wound repair by inducing premature cellular senescence: role for p38 mitogen-activated protein kinase. Burns 35:500–508
Zhou F, Onizawa S, Nagai A et al (2011) Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res 12:78
Buchner N, Ale-Agha N, Jakob S et al (2013) Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp Gerontol 48:8–16
Aoshiba K, Zhou F, Tsuji T et al (2012) DNA damage as a molecular link in the pathogenesis of COPD in smokers. Eur Respir J 39:1368–1376
Kreiling JA, Tamamori-Adachi M, Sexton AN et al (2011) Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell 10:292–304
Wang C, Jurk D, Maddick M et al (2009) DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8:311–323
Shivshankar P, Boyd AR, Le Saux CJ et al (2011) Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell 10:798–806
d’Adda di Fagagna F, Reaper PM, Clay-Farrace L et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198
Tsuji T, Aoshiba K, Nagai A (2004) Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol 31:643–649
Kim CO, Huh AJ, Han SH et al (2012) Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch Gerontol Geriatr 54:e35–e41
Muller M (2006) Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic Biol Med 41:1670–1677
Aoshiba K, Tsuji T, Nagai A (2003) Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J 22:436–443
Nyunoya T, Monick MM, Klingelhutz AL et al (2009) Cigarette smoke induces cellular senescence via Werner’s syndrome protein down-regulation. Am J Respir Crit Care Med 179:279–287
Hara H, Araya J, Takasaka N et al (2012) Involvement of creatine kinase B in cigarette smoke-induced bronchial epithelial cell senescence. Am J Respir Cell Mol Biol 46:306–312
Fujii S, Hara H, Araya J et al (2012) Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology 1:630–641
Amsellem V, Gary-Bobo G, Marcos E et al (2011) Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184:1358–1366
Minagawa S, Araya J, Numata T et al (2011) Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300:L391–L401
Savale L, Chaouat A, Bastuji-Garin S et al (2009) Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179:566–571
Houben JM, Mercken EM, Ketelslegers HB et al (2009) Telomere shortening in chronic obstructive pulmonary disease. Respir Med 103:230–236
Mui TS, Man JM, McElhaney JE et al (2009) Telomere length and chronic obstructive pulmonary disease: evidence of accelerated aging. J Am Geriatr Soc 57:2372–2374
Alder JK, Chen JJ, Lancaster L et al (2008) Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A 105:13051–13056
Cronkhite JT, Xing C, Raghu G et al (2008) Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med 178:729–737
Holz O, Zuhlke I, Jaksztat E et al (2004) Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J 24:575–579
Parker SM, Goriwiec MR, Borthwick LA et al (2008) Airway epithelial cell senescence in the lung allograft. Am J Transplant 8:1544–1549
Fukuchi Y (2009) The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc 6:570–572
Ito K, Barnes PJ (2009) COPD as a disease of accelerated lung aging. Chest 135:173–180
Faner R, Rojas M, Macnee W et al (2012) Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186:306–313
Kasagi S, Seyama K, Mori H et al (2006) Tomato juice prevents senescence-accelerated mouse P1 strain from developing emphysema induced by chronic exposure to tobacco smoke. Am J Physiol Lung Cell Mol Physiol 290:L396–L404
Londhe VA, Sundar IK, Lopez B et al (2011) Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res 69:371–377
Müller KC, Welker L, Paasch K et al (2006) Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res 7:32
Waisberg DR, Parra ER, Barbas-Filho JV et al (2012) Increased fibroblast telomerase expression precedes myofibroblast alpha-smooth muscle actin expression in idiopathic pulmonary fibrosis. Clinics (Sao Paulo) 67:1039–1046
Diaz de Leon A, Cronkhite JT, Katzenstein AL et al (2010) Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One 5:e10680
Naik PK, Moore BB (2011) Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med 4:759–771
Linge A, Weinhold K, Blasche R et al (2007) Downregulation of caveolin-1 affects bleomycin-induced growth arrest and cellular senescence in A549 cells. Int J Biochem Cell Biol 39:1964–1974
Volonte D, Zhang K, Lisanti MP et al (2002) Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell 13:2502–2517
Shivshankar P, Brampton C, Miyasato S et al (2012) Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol 47:28–36
Fridlender ZG, Cohen PY, Golan O et al (2007) Telomerase activity in bleomycin-induced epithelial cell apoptosis and lung fibrosis. Eur Respir J 30:205–213
Liu T, Chung MJ, Ullenbruch M et al (2007) Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest 117:3800–3809
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130:223–233
Bartling B, Desole M, Silber RE et al (2007) Dicarbonyl-mediated protein modifications affect matrix metalloproteinase (MMP) activity. Z Gerontol Geriatr 40:357–361
Bartling B, Rehbein G, Silber RE et al (2006) Senescent fibroblasts induce moderate stress in lung epithelial cells in vitro. Exp Gerontol 41:532–539
Ito K, Colley T, Mercado N (2012) Geroprotectors as a novel therapeutic strategy for COPD, an accelerating aging disease. Int J Chron Obstruct Pulmon Dis 7:641–652
Noureddine H, Gary-Bobo G, Alifano M et al (2011) Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res 109:543–553
Tsuji T, Aoshiba K, Nagai A (2006) Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174:886–893
Tsuji T, Aoshiba K, Nagai A (2010) Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration 80:59–70
Hosokawa M, Fujisawa H, Ax S et al (2000) Age-associated DNA damage is accelerated in the senescence-accelerated mice. Mech Ageing Dev 118:61–70
Albino AP, Huang X, Jorgensen E et al (2004) Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: a new assay for carcinogens. Cell Cycle 3:1062–1068
Tanaka T, Huang X, Jorgensen E et al (2007) ATM activation accompanies histone H2AX phosphorylation in A549 cells upon exposure to tobacco smoke. BMC Cell Biol 8:26
Rammah M, Dandachi F, Salman R et al (2012) In vitro cytotoxicity and mutagenicity of mainstream waterpipe smoke and its functional consequences on alveolar type II derived cells. Toxicol Lett 211:220–231
Klimova TA, Bell EL, Shroff EH et al (2009) Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J 23:783–794
Compliance with ethical guidelines
Conflict of interest. B. Bartling states that there are no conflicts of interests.
The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartling, B. Cellular senescence in normal and premature lung aging. Z Gerontol Geriat 46, 613–622 (2013). https://doi.org/10.1007/s00391-013-0543-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00391-013-0543-3