Abstract
Objectives
Our goal was to estimate the diagnostic accuracy of substantia nigra fractional anisotropy (SN-FA) for Parkinson’s disease (PD) diagnosis in a sample similar to the clinical setting, including patients with essential tremor (ET) and healthy controls (HC). We also performed a systematic review and meta-analysis to estimate mean change in SN-FA induced by PD and its diagnostic accuracy.
Methods
Our sample consisted of 135 subjects: 72 PD, 21 ET and 42 HC. To address inter-scanner variability, two 3.0-T MRI scans were performed. MRI results of this sample were pooled into a meta-analysis that included 1,432 subjects (806 PD and 626 HC). A bivariate model was used to evaluate diagnostic accuracy measures.
Results
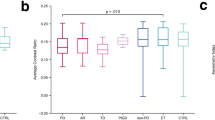
In our sample, we did not observe a significant effect of disease on SN-FA and it was uninformative for diagnosis. The results of the meta-analysis estimated a 0.03 decrease in mean SN-FA in PD relative to HC (CI: 0.01–0.05). However, the discriminatory capability of SN-FA to diagnose PD was low: pooled sensitivity and specificity were 72 % (CI: 68–75) and 63 % (CI: 58–70), respectively. There was high heterogeneity between studies (I2 = 91.9 %).
Conclusions
SN-FA cannot be used as an isolated measure to diagnose PD.
Key Points
• SN-FA appears insufficiently sensitive and specific to diagnose PD.
• Radiologists must be careful when translating mean group results to clinical practice.
• Imaging protocol and analysis standardization is necessary for developing reproducible quantitative biomarkers.




Similar content being viewed by others

References
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870
Marquand AF, Filippone M, Ashburner J et al (2013) Automated, high accuracy classification of parkinsonian disorders: a pattern recognition approach. PLoS ONE 8:e69237
Shih MC, Amaro E, Ferraz HB et al (2006) Neuroimaging of the dopamine transporter in Parkinsons disease: first study using [99mTc]-TRODAT-1 and SPECT in Brazil. Arq Neuropsiquiatr 64:628–634
Vaillancourt DE, Spraker MB, Prodoehl J et al (2009) High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 72:1378–1384
Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP (2013) Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: a region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage Clin 3:481–488
Cochrane CJ, Ebmeier KP (2013) Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology 80:857–864
Obuchowski NA, McClish DK (1997) Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med 16:1529–1542
Raunig DL, McShane LM, Pennello G et al (2015) Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res 24:27–67
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982–990
Chan LL, Rumpel H, Yap K et al (2007) Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78:1383–1386
Menke RA, Jbabdi S, Miller KL, Matthews PM, Zarei M (2010) Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson’s disease. NeuroImage 52:1175–1180
Péran P, Cherubini A, Assogna F et al (2010) Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 133:3423–3433
Rolheiser TM, Fulton HG, Good KP et al (2011) Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol 258:1254–1260
Wang JJ, Lin WY, Lu CS et al (2011) Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology 261:210–217
Zhan W, Kang GA, Glass GA et al (2012) Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Mov Disord 27:90–97
Prakash BD, Sitoh Y-Y, Tan LC, Au WL (2012) Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson’s disease. Parkinsonism Relat Disord 18:1029–1033
Du G, Lewis MM, Styner M et al (2011) Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov Disord 26:1627–1632
Skorpil M, Söderlund V, Sundin A, Svenningsson P (2012) MRI diffusion in Parkinson“s disease: using the technique”s inherent directional information to study the olfactory bulb and substantia nigra. J Parkinsons Dis 2:171–180
Scherfler C, Esterhammer R, Nocker M et al (2013) Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson’s disease. Brain 136:3028–3037
Perea RD, Rada RC, Wilson J et al (2013) A comparative white matter study with Parkinson’s disease, Parkinson’s disease with dementia and Alzheimer’s disease. J Alzheimers Dis Parkinsonism 3:123
Chan L-L, Ng K-M, Rumpel H, Fook-Chong S, Li HH, Tan EK (2014) Transcallosal diffusion tensor abnormalities in predominant gait disorder parkinsonism. Parkinsonism Relat Disord 20:53–59
Lenfeldt N, Larsson A, Nyberg L, Birgander R, Forsgren L (2015) Fractional anisotropy in the substantia nigra in Parkinson’s disease: a complex picture. Eur J Neurol 22:1408–1414
Jiang M-F, Shi F, Niu G-M, Xie SH, Yu SY (2015) A novel method for evaluating brain function and microstructural changes in Parkinson’s disease. Neural Regen Res 10:2025–2028
Schuff N, Wu I-W, Buckley S et al (2015) Diffusion imaging of nigral alterations in early Parkinson’s disease with dopaminergic deficits. Mov Disord 30:1885–1892
Zhang G, Zhang Y, Zhang C et al. Research article diffusion kurtosis imaging of substantia nigra is a sensitive method for early diagnosis and disease evaluation in Parkinson’s disease. Parkinson’s disease. Hindawi Publishing Corporation. 2015; Article ID 207624: 1–5 doi: 10.1155/2015/207624
Langley J, Huddleston DE, Merritt M et al (2016) Diffusion tensor imaging of the substantia nigra in Parkinson’s disease revisited. Hum Brain Mapp 37:2547–2556
Nagae LM, Honce JM, Tanabe J, Shelton E, Sillau SH, Berman BD (2016) Microstructural changes within the basal ganglia differ between Parkinson disease subtypes. Front Neuroanat 10:17
Loane C, Politis M, Kefalopoulou Z et al (2016) Aberrant nigral diffusion in Parkinson’s disease: a longitudinal diffusion tensor imaging study. Mov Disord 31:1020–1026
Kamagata K, Hatano T, Okuzumi A et al (2016) Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur Radiol 26:2567–2577
Jones DK (2004) The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med 51:807–815
Jones DK, Knösche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage 73:239–254
Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B (2014) Spurious group differences due to head motion in a diffusion MRI study. NeuroImage 88:79–90
Jellinger K, Kienzl E, Rumpelmair G, Riederer P, Stachelberger H, Ben-Shachar D, Youdim MB (1992) Iron-melanin complex in substantia nigra of parkinsonian brains: an x-ray microanalysis. J Neurochem 59:1168–1171
Zhang J, Tao R, Liu C et al (2013) Possible effects of iron deposition on the measurement of DTI metrics in deep gray matter nuclei: an in vitro and in vivo study. Neurosci Lett 551:47–52
Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S (2007) Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. NeuroImage 36:1123–1138
Bisdas S, Bohning DE, Besenski N, Nicholas JS, Rumboldt Z (2008) Reproducibility, interrater agreement, and age-related changes of fractional anisotropy measures at 3T in healthy subjects: effect of the applied b-value. AJNR Am J Neuroradiol 29:1128–1133
Jones DK, Basser PJ (2004) “Squashing peanuts and smashing pumpkins”: how noise distorts diffusion-weighted MR data. Magn Reson Med 52:979–993
Teipel SJ, Reuter S, Stieltjes B et al (2011) Multicenter stability of diffusion tensor imaging measures: a European clinical and physical phantom study. Psychiatry Res 194:363–371
Pepe MS (2003) The statistical evaluation of medical tests for classification and prediction. Oxford University Press, Oxford
Cosottini M, Frosini D, Pesaresi I et al (2014) MR imaging of the Substantia Nigra at 7 T enables diagnosis of Parkinson disease. Radiology 271:831–838
Blazejewska AI, Schwarz ST, Pitiot A et al (2013) Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology 81:534–540
Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP (2014) The “swallow tail” appearance of the healthy Nigrosome – A new accurate test of Parkinson’s disease: a case-control and retrospective cross-sectional MRI Study at 3T. PLoS One 9:e93814
Kim J-M, Jeong H-J, Bae YJ et al (2016) Loss of substantia nigra hyperintensity on 7 Tesla MRI of Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Parkinsonism Relat Disord 26:47–54
Acknowledgments
The authors thank the Brazil Parkinson-Association for helping to contact volunteers during the recruitment phase; Adelinda da Silva Arruda Gonçalves, Karina Fernandes Dias Correa e Alda Fernandes Castro for administrative support; and Dr. Marcelo Buarque Gusmão Funari, Head of the Radiology Department of the Hospital Israelita Albert Einstein for supporting this study. We also thank the R-Community for providing and maintaining a free software environment for statistical computing and graphics and full financial support from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
The scientific guarantor of this publication is Ellison Fernando Cardoso. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received support from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) - Grant number: 2011/18747. Three of the authors have significant statistical expertise (João Ricardo Sato, Gilson Vieira and Ellison Fernando Cardoso). Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects in this study. Methodology: prospective, diagnostic study, multicentre study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 8.44 mb)
Rights and permissions
About this article
Cite this article
Hirata, F.C.C., Sato, J.R., Vieira, G. et al. Substantia nigra fractional anisotropy is not a diagnostic biomarker of Parkinson’s disease: A diagnostic performance study and meta-analysis. Eur Radiol 27, 2640–2648 (2017). https://doi.org/10.1007/s00330-016-4611-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4611-0