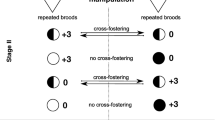
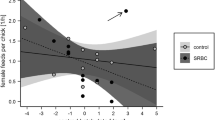
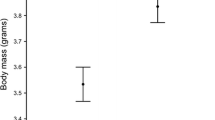
Abstract
Hormone-mediated maternal effects play an important role in the formation of a differentiated phenotype. They have been shown to influence a wide array of offspring traits, both early in life and in adulthood. One important offspring trait that is under the influence of maternal androgens is the immune system. In birds, a growing number of studies show that yolk androgens modulate immune function during the chick stage. However, there is a lack of knowledge regarding long-term effects of prenatal androgens on offspring immunity. In this study, we therefore investigated the influence of prenatal testosterone (T) on several measures of immunity in fledgling and adult zebra finches (Taeniopygia guttata). Cell-mediated immune response (towards phytohaemagglutinin, PHA) of fledglings hatching from control eggs was negatively related to brood size, whereas there was no such association for fledglings hatching from eggs with experimentally elevated T levels (T fledglings). Male control fledglings showed reduced mass gain compared to female control fledglings within 24 h after the PHA injection. This pattern was reversed in T fledglings. Total antibody levels in fledglings were not affected by egg treatment. Neither cell-mediated immunity nor total antibody levels in sexually mature zebra finches were influenced by egg treatment. However, there was an immuno-enhancing effect of elevated egg T on both primary and secondary humoral immune responses toward diphtheria and tetanus antigens in ca 5 and 7 month old zebra finches. In addition, the covariation between different immune components differed between T and control offspring, suggesting that egg treatment may have altered the potential trade-offs between different parts of the immune system. Our results suggest that prenatal androgens could be an important factor contributing to individual variation in immune function even in adulthood.




Similar content being viewed by others
References
Adamo SA (2004) How should behavioural ecologists interpret measurements of immunity? Anim Behav 68:1443–1449
Andersson S, Uller T, Lohmus M, Sundström F (2004) Effects of egg yolk testosterone on growth and immunity in a precocial bird. J Evol Biol 17: 501–505
Ardia DR (2007) The ability to mount multiple immune responses simultaneously varies across the range of the tree swallow. Ecography 30:23–30
Arriero E (2009) Rearing environment effects on immune defence in blue tit Cyanistes caerulus nestlings. Oecologia 159:697–704
Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G (2003) Assessing the cost of mounting an immune response. Am Nat 161:367–379
Buchanan KL, Evans MR, Goldsmith AR (2003) Testosterone, dominance signalling and immunosupression in the house sparrow, Passer domesticus. Behav Ecol Sociobiol 55:50–59
Carere C, Balthazart J (2007) Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrin Metab 18:73–80
Clark MM, Galef J (1995) Prenatal influences on reproductive life history strategies. Trends Ecol Evol 10:151–153
Cucco M, Guasco B, Malacarne G, Ottonelli R, Tanvez A (2008) Yolk testosterone levels and dieatary carotenoids influence growth and immunity of grey partridge chicks. Gen Comp Endocrinol 156:418–425
Eising C, Müller W, Groothuis TGG (2006) Avian mothers create different phenotypes by hormone deposition in their eggs. Biol Lett 2:20–22
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman, Essex
Gil D, Graves J, Hazon N, Wells A (1999) Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286:126–128
Gil D (2008) Hormones in Avian Eggs: Physiology, Ecology and Behavior. Advances in the Study of Behavior 38:337–398
Grindstaff J, Hasselquist D, Nilsson J-A, Sandell M, Smith H, Stjernman M (2006) Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc Roy Soc Lond B 273:2551–2557
Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C (2005a) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352
Groothuis TGG, Eising C, Dijkstra C, Müller W (2005b) Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol Lett 1:78–81
Hasselquist D (2007) Comparative immunoecology in birds: hypotheses and tests. J Ornithol 148:S571–S582
Hasselquist D, Marsh JA, Sherman PW, Wingfield JC (1999) Is avian humoral immunocompetence suppressed by testosterone? Behav Ecol Sociobiol 45:167–175
Hasselquist D, Wasson MF, Winkler DW (2001) Humoral immunocompetence correlates with date of egg-laying and reflects work load in female tree swallows. Behav Ecol 12:93–97
Horak P, Tegelmann L, Ots I, Moller AP (1999) Immune function and survival of great tit nestlings in relation to growth conditions. Oecologia 121:316–322
Ilmonen P, Hasselquist D, Langefors Å, Wiehn J (2003) Stress, immunocompetence and leukocyte profiles of pied flycatchers in relation to brood size manipulation. Oecologia 136:148–154
Janeway CA, Travers P (1996) Immunobiology: The immune system in health and disease. Garland Publishing, New York
Kelly C, Price T (2005) Correcting for regression to the mean in behavior and ecology. Am Nat 166:700–707
Klein SL, Nelson RJ (1999) Social interactions unmask sex differences in humoral immunity in voles. Anim Behav 57:603–610
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (2004) SAS System for Mixed Models, 6th edn., Cary, NC: SAS Institute.
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
LoveOP SKG, Dale J, Williams TD (2008) Sex-Specific Variability in the Immune System across Life-History Stages. Am Nat 172:E99–E112
Martin LB, Weil ZM, Kuhlman JR, Nelson RJ (2006a) Trade-offs within the immune systems of female white-footed mice, Peromyscus leucopus. Func Ecol 20:630–636
Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M (2006b) Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Func Ecol 20:290–299
Matson KD, Cohen AA, Klasing KC, Ricklefs RE, Scheuerlein A (2006) No simple answers for ecological immunology: relationships among immune indices at the individual level break down at the species level in water fowl. Proc Roy Soc Lond B 273:815–822
McCormick MI (1999) Experimental test of the effect of maternal hormones on larval quality of a coral reef fish. Oecologia 118:412–422
McKean KA, Yourth CP, Lazzaro BP, Clark AG (2008) The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol 8:76
Mousseau TA, Fox CW (1998) Maternal effects as adaptations, 1st edn. Oxford University Press, Inc., New York
Müller W, Groothuis TGG, Kasprzik A, Dijkstra C, Alatalo RV, Siitari H (2005) Prenatal androgen exposure modulates cellular and humoral immune function of black-headed gull chicks. Proc Roy SocLond B 272:1971–1977
Müller W, Deptuch K, Lopez-Rull I, Gil D (2007) Elevated yolk androgen levels benefit offspring development in a between-clutch context. Behav Ecol 18:929–936
Müller W, Vergauwen J, Eens M (2008) Yolk testosterone, postnatal growth and song in male canaries. Horm Behav 54:125–133
Müller W, Eens M (2009) Elevated yolk androgen levels and the expression of multiple sexually selected male characters. Horm Behav 55:175–181
Müller W, Vergauwen J, Eens M (2009) Long-lasting consequences of elevated yolk testosterone levels on female reproduction. Behav Ecol Sociobiol 63:809–816
Navara KJ, Hill GE, Mendonca MT (2005) Variable effects of yolk androgens on growth, survival, and immunity in eastern bluebird nestlings. Physiol Biochem Zool 78:570–578
Navara KJ, Hill GE, Mendonca MT (2006) Yolk testosterone stimulates growth and immunity in house finch chicks. Physiol Biochem Zool 79:550–555
Nilsson JF (2009) Causes and consequences of individual variation in energy turnover rates. PhD thesis. Lund University, Sweden
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Owen-Ashley NT, Hasselquist D, Wingfield JC (2004) Androgens and the Immunocompetence Handicap Hypothesis: Unraveling Direct and Indirect Pathways of Immunosuppression in Song Sparrows. Am Nat 164:490–505
Partecke J, Schwabl H (2008) Organizational effects of maternal testosterone on reproductive behavior of adult house sparrows. Devel Neurobiol 68:1538–1548
Pilz KM, Quiroga M, Schwabl H, Adkins-Regan E (2004) European starling chicks benefit from high yolk testosterone levels during a drought year. Horm Behav 46:179–192
Pitala N, Ruuskanen S, Laaksonen T, Doligez B, Tschirren B, Gustafsson L (2009) The effects of experimentally manipulated yolk androgens on growth and immune function of male and female nestling collared flycatchers Ficedula albicollis. J Avian Biol 40:225–230
Råberg L, Stjernman M (2003) Natural selection on immune responsiveness in blue tits Parus caeruleus. Evolution 57:1670–1678
Roberts ML, Buchanan KL, Hasselquist D, Evans MR (2007) Effects of testosterone and corticosterone on immunocompetence in the zebra finch. Horm Behav 51:126–134
Roberts M, Peters A (2009) Is testosterone immunosuppressive in a condition-dependent manner? An experimental test in blue tits. J Exp Biol 212:1811–1818
Rubolini D, Romano M, Martinelli R, Leoni B, Saino N (2006a) Effects of prenatal yolk androgens on armaments and ornaments of the ring-necked pheasant. Behav Ecol Sociobiol 59:549–560
Rubolini D, Romano M, Martinelli R, Saino N (2006b) Effects of elevated yolk testosterone on survival, growth and immunity of male and female yellow-legged gull chicks. Behav Ecol Sociobiol 59:344–352
Rubolini D, Martinelli R, von Engelhardt N, Romano M, Groothuis TGG, Fasola M, Saino N (2007) Consequences of prenatal androgen exposure for the reproductive performance of female pheasants (Phasanius colchicus). Proc Roy Soc Lond B 274:137–142
Sadd BM, Schmid-Hempel P (2008) Principles of ecological immunology. Evol Appl 2:113–121
Saino N, Calza S, Moller AP (1997) Immunocompetence of nestling barn swallows in relation to brood size and parental effort. J Anim Ecol 66:827–836
Sandell MI, Tobler M, Hasselquist D (2009) Yolk androgens and the development of avian immunity: an experiment in jackdaws (Corvus monedula). J Exp Biol 212:815–822
Schmid-Hempel P (2003) Variation in immune defence as a question of evolutionary ecology. Proc Roy Soc Lond B 270:357–366
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Simmons LW, Roberts B (2005) Bacterial immunity traded for sperm viability in male crickets. Science 309:2031
Smits JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Func Ecol 13:567–572
Stearns SC (2003) The evolution of life histories, 7th edn. Oxford University Press, New York
Strasser R, Schwabl H (2004) Yolk testosterone organizes behaviour and male plumage coloration in house sparrows (Passer domesticus). Behav Ecol Sociobiol 56:491–497
Tobler M, Sandell MI (2007) Yolk testosterone modulates persistence of neophobic responses in adult zebra finches, Taeniopygia guttata. Horm Behav 52:640–645
Tobler M, Sandell MI (2009) Sex-specific effects of prenatal testosterone on nestling plasma antioxidant capacity in the zebra finch. J Exp Biol 212:89–94
Tschirren B, Saladin V, Fitze PS, Schwabl H, Richner H (2005) Maternal yolk testosterone does not modulate parasite susceptibility or immune function in great tit nestlings. J Anim Ecol 74:675–682
Uller T, Eklöf J, Andersson S (2005) Female egg investment in relation to male sexual traits and the potential for transgenerational effects in sexual selection. Behav Ecol Sociobiol 57:584–590
Uller T, Olsson M (2003) Prenatal exposure to testosterone increases ectoparasite susceptibility in the common lizard (Lacerta vivipara). Proc Roy Soc Lond B 270:1867–1870
von Engelhardt N, Carere C, Dijkstra C, Groothuis TGG (2006) Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc Roy Soc Lond B 273:65–70
von Engelhardt N (2004) Proximate control of Avian sex allocation, a study in Zebra Finches. PhD thesis. University of Groningen, The Netherlands
Zann RA (1996) The Zebra Finch. A Synthesis of field and laboratory studies. Oxford University Press Inc, Oxford
Zera AJ, Harshman LG (2001) The physiology of life history trade-offs in animals. Ann Rev Ecol Syst 32:95–126
Acknowledgements
We thank Sandra Chiriac for help with the zebra finches. Two anonymous reviewers provided constructive comments which improved the quality of the manuscript. This study was supported by the Royal Physiographic Society in Lund (to MT), the Swedish Research Council for Environment, Agricultural Science and Spatial Planning (Formas) (to DH) and the Swedish Research Council (to DH and MIS). The experiment was performed with approval from the Malmö/Lund ethical committee for animal research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Graves
Rights and permissions
About this article
Cite this article
Tobler, M., Hasselquist, D., Smith, H.G. et al. Short- and long-term consequences of prenatal testosterone for immune function: an experimental study in the zebra finch. Behav Ecol Sociobiol 64, 717–727 (2010). https://doi.org/10.1007/s00265-009-0889-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0889-0