Abstract
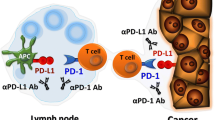
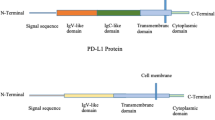
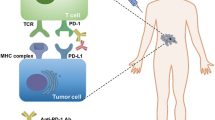
Immunotherapy has moved to the center stage of cancer treatment with the recent success of trials in solid tumors with PD-1/PD-L1 axis blockade. Programmed death-1 or PD-1 is a checkpoint molecule on T cells that plays a vital role in limiting adaptive immune responses and preventing autoimmune and auto-inflammatory reactivity in the normal host. In cancer patients, PD-1 expression is very high on T cells in the tumor microenvironment, and PD-L1, its primary ligand, is variably expressed on tumor cells and antigen-presenting cells within tumors, providing a potent inhibitory influence within the tumor microenvironment. While PD-L1 expression on tumors is often regarded as a negative prognostic factor, it is clearly associated with a positive outcome for treatment with PD-1/PD-L1 blocking antibodies, and has been used to select patients for this therapy. Responses of long duration, a minority of patients with atypical responses in which progression may precede tumor shrinkage, and a pattern of autoimmune side effects often seen with this class of drugs characterize therapy with PD-1/PD-L1 blocking drugs. While excellent efficacy has been seen with a limited number of tumor types, most epithelial cancers do not show responses of long duration with these agents. In the current review, we will briefly summarize the scientific background data supporting the development of PD-1/PD-L1 blockade, and then describe the track record of these antibodies in multiple different histologies ranging from melanoma and lung cancer to less common tumor types as well as discuss biomarkers that may assist in patient selection.
Similar content being viewed by others
Abbreviations
- AACR:
-
American Association of Cancer Research
- ASCO:
-
American Society of Clinical Oncology
- BCG:
-
Bacillus Calmette–Guerin
- CI:
-
Confidence interval
- CR:
-
Complete response
- CTLA-4:
-
Cytotoxic T-lymphocyte associated protein-4
- DCR:
-
Disease control rate (ORR and SD)
- FKSI-DRS:
-
Functional assessment of cancer therapy–kidney symptom index–disease-related symptoms
- GVHD:
-
Graft-versus-host disease
- HCT:
-
Hematopoietic stem cell transplant
- HL:
-
Hodgkin’s lymphoma
- IC:
-
Immune cell
- IDO1:
-
Indoleamine 2,3 dioxygenase 1
- IHC:
-
Immunohistochemistry
- MRD:
-
Mismatch repair deficiency
- ORR:
-
Objective response rate
- PCR:
-
Polymerase chain reaction
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death ligand-1
- PD-L2:
-
Programmed death ligand-2
- PTEN:
-
Phosphatase and tensin homolog
- SD:
-
Stable disease
References
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11(11):3887–3895
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T (1996) Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8(5):765–772
Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger LR (1997) Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp Cell Res 232(1):25–28. doi:10.1006/excr.1997.3493
Nishimura H, Nose M, Hiai H, Minato N, Honjo T (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11(2):141–151
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439(7077):682–687. doi:10.1038/nature04444
Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443(7109):350–354. doi:10.1038/nature05115
Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, Weber JS (2007) Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol 19(10):1223–1234. doi:10.1093/intimm/dxm091
Wu K, Kryczek I, Chen L, Zou W, Welling TH (2009) Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 69(20):8067–8075. doi:10.1158/0008-5472.CAN-09-0901
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA (2009) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114(8):1537–1544. doi:10.1182/blood-2008-12-195792
Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM (2010) Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207(10):2175–2186. doi:10.1084/jem.20100637
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K (2010) Tumor-infiltrating NY-ESO-1-specific CD8 + T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA 107(17):7875–7880. doi:10.1073/pnas.1003345107
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4(127):127ra137. doi:10.1126/scitranslmed.3003689
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF (2013) Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 5(200):200ra116. doi:10.1126/scitranslmed.3006504
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13(1):84–88. doi:10.1038/nm1517
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G (2014) PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2(4):361–370. doi:10.1158/2326-6066.CIR-13-0127
Chen L, Han X (2015) Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 125(9):3384–3391. doi:10.1172/JCI80011
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ (2007) Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27(1):111–122. doi:10.1016/j.immuni.2007.05.016
Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, Liu Y, Strome SE, Chen L, Tamada K (2010) B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 116(8):1291–1298. doi:10.1182/blood-2010-01-265975
Dong H, Chen L (2003) B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med (Berl) 81(5):281–287. doi:10.1007/s00109-003-0430-2
Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L (2008) B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 111(7):3635–3643. doi:10.1182/blood-2007-11-123141
Ahmad SM, Borch TH, Hansen M, Andersen MH (2016) PD-L1-specific T cells. Cancer Immunol Immunother 65(7):797–804. doi:10.1007/s00262-015-1783-4
Kamata T, Suzuki A, Mise N, Ihara F, Takami M, Makita Y, Horinaka A, Harada K, Kunii N, Yoshida S, Yoshino I, Nakayama T, Motohashi S (2016) Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother 65(12):1477–1489. doi:10.1007/s00262-016-1901-y
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28(19):3167–3175. doi:10.1200/JCO.2009.26.7609
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. doi:10.1056/NEJMoa1200690
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32(10):1020–1030. doi:10.1200/JCO.2013.53.0105
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. doi:10.1056/NEJMoa1412082
Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA (2013) Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 31(34):4311–4318. doi:10.1200/JCO.2013.51.4802
Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16(4):375–384. doi:10.1016/S1470-2045(15)70076-8
Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, Wolchok JD (2015) Efficacy and Safety of Nivolumab in patients with BRAF V600 mutant and BRAF Wild-Type Advanced Melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol 1(4):433–440. doi:10.1001/jamaoncol.2015.1184
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369(2):134–144. doi:10.1056/NEJMoa1305133
Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, Patnaik A, Dronca R, Zarour H, Joseph RW, Boasberg P, Chmielowski B, Mateus C, Postow MA, Gergich K, Elassaiss-Schaap J, Li XN, Iannone R, Ebbinghaus SW, Kang SP, Daud A (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384(9948):1109–1117. doi:10.1016/S0140-6736(14)60958-2
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, Cranmer LD, Blank CU, O’Day SJ, Ascierto PA, Salama AK, Margolin KA, Loquai C, Eigentler TK, Gangadhar TC, Carlino MS, Agarwala SS, Moschos SJ, Sosman JA, Goldinger SM, Shapira-Frommer R, Gonzalez R, Kirkwood JM, Wolchok JD, Eggermont A, Li XN, Zhou W, Zernhelt AM, Lis J, Ebbinghaus S, Kang SP, Daud A (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16(8):908–918. doi:10.1016/S1470-2045(15)00083-2
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K- (2015) Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372(26):2521–2532. doi:10.1056/NEJMoa1503093
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133. doi:10.1056/NEJMoa1302369
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372(21):2006–2017. doi:10.1056/NEJMoa1414428
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. doi:10.1056/NEJMoa1504030
Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL Jr, Lawrence DP, Logan TF, Schuchter LM, Nair S, Fecher L, Buchbinder EI, Berghorn E, Ruisi M, Kong G, Jiang J, Horak C, Hodi FS (2016) Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 17(7):943–955. doi:10.1016/S1470-2045(16)30126-7
Hodi FS PM, Chesney JA, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala S, Shaheen MF, Ernstoff MS, Minor DR, Salama AK, Taylor MH, Ott PA, Jiang J, Gagnier P, Wolchok JD (2016) Overall survival in patients with advanced melanoma (MEL) who discontinued treatment with nivolumab (NIVO) plus ipilimumab (IPI) due to toxicity in a phase II trial (CheckMate 069). In: ASCO Annual Meeting, Chicago, IL, 2016. J Clin Oncol, vol 34 (suppl; abstr 9518)
Hodi FS KH, Sznol M, Carvajal R, Lawrence D, Atkins M, Powderly J, Sharfman W, Puzanov I, Smith D, Leming P, Lipson E, Taube J, Anders R, Horak C, Jiang J, McDermott D, Sosman J, Brahmer J, Pardoll D, Topalian S (2016) Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial In: 107th Annual Meeting of the American Association for Cancer Research, New Orleans, LA, April 16–20, 2016
Robert C RA, Hamid O, Daud A, Wolchok JD, Joshua AM, Hwu W-J, Weber JS, Gangadhar TC, Joseph RJ, Dronca RS, Patnaik A, Zarour HM, Kefford R, Hersey P, Li X, Diede SJ, Ebbinghaus S, Hodi FS (2016) Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. In: ASCO Annual Meeting, Chicago, IL, 2016. J Clin Oncol, vol 34 (suppl; abstr 9503)
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD (2015) Pooled analysis of long-term survival data from phase II and phase III trials of Ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33(17):1889–1894. doi:10.1200/JCO.2014.56.2736
Long GV DR, Ribas A, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon JS, Fernandez E, Kirkwood JM, Thomas Gajewski T, Christine K, Gause CK, Chen L, Gorski K, Anderson A, Kaufman DR, Chou J, Hodi FS (2016) Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. In: ASCO Annual Meeting, Chicago, IL, 2016
Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR (2015) Overall survival and long-term safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33(18):2004–2012. doi:10.1200/JCO.2014.58.3708
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. doi:10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. doi:10.1056/NEJMoa1504627
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K- (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028. doi:10.1056/NEJMoa1501824
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet 387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567. doi:10.1038/nature14011
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A, Group PS (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet 387(10030):1837–1846. doi:10.1016/S0140-6736(16)00587-0
Barlesi FPK, Ciardiello F, Pawel JV, Gadgeel S, Hida T, Kowalski D, Dols MC, Cortinovis D, Leach J, Polikoff J, Gandara D, Barrios CH, Chen DS, He P, Kowanetz M, Ballinger M, Waterkamp D, Sandler A, Rittmeyer A (2016) Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. In: ESMO Congress, Copenhagen, Denmark, 2016. Vol LBA 44
Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, Juergens RA, Laurie SA, Nathan FE, Shen Y, Harbison CT, Hellmann MD (2016) Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 34(25):2980–2987. doi:10.1200/JCO.2016.66.9929
Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie SA, Goldman JW, Shepherd FA, Chen AC, Shen Y, Nathan FE, Harbison CT, Antonia S (2016) Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 34(25):2969–2979. doi:10.1200/JCO.2016.66.9861
Company B-MS (2016) Bristol-Myers Squibb announces top-line results from CheckMate-026, a phase 3 study of Opdivo (nivolumab) in treatment-naïve patients with advanced non-small cell lung cancer
Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, Winquist E, Culine S, von der Maase H, Vaughn DJ, Rosenberg JE (2010) Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28(11):1850–1855. doi:10.1200/JCO.2009.25.4599
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van ‘t Veer L, Vincent-Salomon A, Waddell N, Yates LR; Australian Pancreatic Cancer Genome I, Consortium IBC, Consortium IM-S, PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR (2013) Signatures of mutational processes in human cancer. Nature 500(7463):415–421. doi:10.1038/nature12477
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515(7528):558–562. doi:10.1038/nature13904
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387(10031):1909–1920. doi:10.1016/S0140-6736(16)00561-4
Dreicer R, Hoffman-Censits J, Flaig T, Grande E, Balmanoukian A, von Amsberg G, Theodore C, Chowdhury S, Bracarda S, Clement JM, Yu E, Kalebasty AR, Niegisch G, Culine S GM, Ding B, Mariathasan S, Legrand F, Abidoye OO, DP P Updated efficacy from IMvigor210: Atezolizumab in platinum-treated locally advanced/metastatic urothelial carcinoma (mUC). In: ASCO Annual Meeting, Chicago, IL, 2016. J Clin Oncol 34, 2016 (suppl; abstr 4515)
Raggi D, Miceli R, Sonpavde G, Giannatempo P, Mariani L, Galsky MD, Bellmunt J, Necchi A (2016) Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol 27(1):49–61. doi:10.1093/annonc/mdv509
Galsky MD, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, Angel Arranz J, Pal S, Ohyama C, Saci A, Lambert A, Krishnan S, Azrilevich A, Sharma P (2016) Efficacy and safety of nivolumab monotherapy in patients with metastatic urothelial cancer (mUC) who have received prior treatment: results from the phase II CheckMate 275 study. In: ESMO Congress, Copenhagen, Denmark, October 7–11, 2016. vol LBA 31
Bellmunt J DWR, Vaughn DJ, Fradet Y, Lee JY, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Puhlmann M, Perini RF, Bajorin DF (2016) KEYNOTE-045: Open-Label, Phase 3 Study of Pembrolizumab vs Investigator’s Choice of Paclitaxel, Docetaxel, or Vinflunine for Previously Treated Advanced Urothelial Cancer. In: SITC Annual Meeting, National Harbor, MD, November 9–13, 2016
De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, Gil T, Marreaud S, Daugaard G, Skoneczna I, Collette S, Lorent J, de Wit R, Sylvester R (2012) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 30(2):191–199. doi:10.1200/JCO.2011.37.3571
Balar A, Galsky M, Loriot Y, Dawson N, Necchi A, Srinivas S, Joseph R, Vaishampayan U, Sridhar S, Quinn D, Drakaki A, Duran I, Rosenberg J, Powles TH-C, JH, Cui N, Mariathasan S, Thastrom A, Abidoye O, Bajorin D (2016) Atezolizumab (atezo) as first-line (1L) therapy in cisplatin-ineligible locally advanced/metastatic urothelial carcinoma (mUC): primary analysis of IMvigor210 cohort 1. In: ASCO Annual Meeting, Chicago, IL, 2016. J Clin Oncol, vol 34, 2016 (suppl; abstr LBA4500)
Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke JJ, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, O’Donnell PH, Drakaki A, Kurland J, Rebelatto MC, Jin X, Blake-Haskins JA, Gupta AK, NH S (2016) Safety and efficacy of durvalumab (MEDI4736), a PD-L1 antibody, in urothelial bladder cancer. In: ASCO Annual Meeting, Chicago, IL, 2016
Apolo AB, Infante JR, Hamid O, Patel MR, Wang D, K K, Mega AE, Britten CD, Ravaud A, Mita AC, Safran H, Stinchcombe T, Grote HJ, von Heydebreck A, Cuillerot JM, JL G (2016) Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: Analysis of safety, clinical activity, and PD-L1 expression. In: ASCO Annual Meeting, Chicago, IL, 2016
Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, Sherry RM, Royal RE, Steinberg SM, Rosenberg S (2008) High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 113(2):293–301. doi:10.1002/cncr.23552
Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack er, lambert am, waxman im, hammers hj (2014) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. doi:10.1200/JCO.2014.59.0703
McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fasso M, Wang YV, Zou W, Hegde PS, Fine GD, Powles T (2016) Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 34(8):833–842. doi:10.1200/JCO.2015.63.7421
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813. doi:10.1056/NEJMoa1510665
Cella D, Grunwald V, Nathan P, Doan J, Dastani H, Taylor F, Bennett B, DeRosa M, Berry S, Broglio K, Berghorn E, Motzer RJ (2016) Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol 17(7):994–1003. doi:10.1016/S1470-2045(16)30125-5
Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, Armand P, Chapuy B, de Jong D, Hoppe RT, Neuberg DS, Rodig SJ, Shipp MA (2016) PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 34(23):2690–2697. doi:10.1200/JCO.2016.66.4482
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372(4):311–319. doi:10.1056/NEJMoa1411087
Mehra RST, Mahipal A, Weiss J, Berger R, Eder JP, Burtness B, Tahara M, Keam B, Le DT, Muro K, Geva R, Chung HC, Lin CC, Meister A, Hille D, Cheng JD, Man Chow LQ, Haddad RI (2016) Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC): pooled analyses after long-term follow-up in KEYNOTE-012. In: ASCO Annual Meeting, 2016
Diaz LA UJ, Wang H, Bartlett B, Kemberling H, Eyring A, Saba Azad N, Dauses T, Laheru D, Lee JJ, Crocenzi TS, Goldberg RM, Fisher GA, Greten TF, Meyer CF, Fader AN, Armstrong DK, Koshiji M, Vogelstein B, Le DT (2016) Programmed death-1 blockade in mismatch repair deficient cancer independent of tumor histology. In: ASCO Annual Meeting, Chicago, IL, 2016
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520. doi:10.1056/NEJMoa1500596
Funt SCA, Yusko E, Vignali M, Benzeno S, Boyd ME, Meredith MM, Kania BE, Cipolla CK, Regazzi AM, Robins H, Iyer G, Rosenberg JE, Bajorin DF (2016) Correlation of peripheral and intratumoral T-cell receptor (TCR) clonality with clinical outcomes in patients with metastatic urothelial cancer (mUC) treated with atezolizumab. In: ASCO Annual Meeting, Chicago, IL, 2016. vol J Clin Oncol 34, 2016 (suppl; abstr 3005)
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571. doi:10.1038/nature13954
Yusko EVM, Wilson R, Mardis E, Hodi S, Horak C, Chang H, Woods DM, Robins H, Weber J (2016) Baseline tumor T cell receptor (TcR) sequencing analysis and neo antigen load is associated with benefit in melanoma patients receiving sequential nivolumab and ipilimumab. In: ESMO Congress, Copenhagen, Denmark, October 7–11, 2016. Abstract 2980
Ratcliffe MJSA, Midha A, Barker C, Scorer P, Walker J (2016) A comparative study of PD-L1 diagnostic assays and the classification of patients as PD-L1 positive and PD-L1 negative. In: AACR Annual Meeting, New Orleans, LA, 2016. LB-094
Gajewski TF (2015) The next hurdle in cancer immunotherapy: overcoming the non-T-cell-inflamed tumor microenvironment. Semin Oncol 42(4):663–671. doi:10.1053/j.seminoncol.2015.05.011
Spranger S, Bao R, Gajewski TF (2015) Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523(7559):231–235. doi:10.1038/nature14404
Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, De Macedo MP, Austin-Breneman JL, Jiang H, Chang Q, Reddy SM, Chen WS, Tetzlaff MT, Broaddus RJ, Davies MA, Gershenwald JE, Haydu L, Lazar AJ, Patel SP, Hwu P, Hwu WJ, Diab A, Glitza IC, Woodman SE, Vence LM, Wistuba, II, Amaria RN, Kwong LN, Prieto V, Davis RE, Ma W, Overwijk WW, Sharpe AH, Hu J, Futreal PA, Blando J, Sharma P, Allison JP, Chin L, Wargo JA (2016) Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 6(8):827–837. doi:10.1158/2159-8290.CD-15-1545
Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, Williams LJ, Deng W, Chen G, Mbofung R, Lazar AJ, Torres-Cabala CA, Cooper ZA, Chen PL, Tieu TN, Spranger S, Yu X, Bernatchez C, Forget MA, Haymaker C, Amaria R, McQuade JL, Glitza IC, Cascone T, Li HS, Kwong LN, Heffernan TP, Hu J, Bassett RL, Jr., Bosenberg MW, Woodman SE, Overwijk WW, Lizee G, Roszik J, Gajewski TF, Wargo JA, Gershenwald JE, Radvanyi L, Davies MA, Hwu P (2016) Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 6(2):202–216. doi:10.1158/2159-8290.CD-15-0283
Sweis RF, Spranger S, Bao R, Paner GP, Stadler WM, Steinberg G, Gajewski TF (2016) Molecular drivers of the non-t-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol Res 4(7):563–568. doi:10.1158/2326-6066.CIR-15-0274
Shastri N, Cardinaud S, Schwab SR, Serwold T, Kunisawa J (2005) All the peptides that fit: the beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol Rev 207:31–41. doi:10.1111/j.0105-2896.2005.00321.x
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371(23):2189–2199. doi:10.1056/NEJMoa1406498
Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Geukes Foppen MH, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA (2015) Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350(6257):207–211. doi:10.1126/science.aad0095
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128. doi:10.1126/science.aaa1348
Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, Algazi AP, Pampaloni MH, Lobach IV, Hwang J, Pierce RH, Gratz IK, Krummel MF, Rosenblum MD (2016) Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 126(9):3447–3452. doi:10.1172/JCI87324
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350(6264):1084–1089. doi:10.1126/science.aac4255
Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350(6264):1079–1084. doi:10.1126/science.aad1329
Zitvogel L, Ayyoub M, Routy B, Kroemer G (2016) Microbiome and anticancer immunosurveillance. Cell 165(2):276–287. doi:10.1016/j.cell.2016.03.001
Derer A, Frey B, Fietkau R, Gaipl US (2016) Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunother 65(7):779–786. doi:10.1007/s00262-015-1771-8
Long GV AV, Cebon JS, Jameson MB, Fitzharris BM, Catriona M, McNeil CM, Hill AG, Ribas A, Atkins MB, Thompson JA, Hwu W-J, Hodi HS, Menzies AM, Guminski AD, Kefford R, Shu X, Ebbinghaus S, Ibrahim N, Carlino MS (2016) Pembrolizumab (pembro) plus ipilimumab (ipi) for advanced melanoma: results of the KEYNOTE-029 expansion cohort. In: ASCO Annual Meeting, Chicago, IL, 2016. J Clin Oncol 34, 2016 (suppl; abstr 9506)
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15(23):7412–7420. doi:10.1158/1078-0432.CCR-09-1624
Acknowledgements
Jeffrey S. Weber has received honoraria from Bristol Myers Squibb, GlaskoSmithKline, Merck, Astra Zeneca, Genentech and EMD Serono, has equity in Celldex, Cytomx and Altor, and has been named on a patent by Moffitt Cancer Center for a biomarker for ipilimumab sensitivity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Arjun Vasant Balar has received honoraria from Merck, Genentech and Astra Zeneca, and has received contracted research support from Merck, Genentech and Astra Zeneca.
Rights and permissions
About this article
Cite this article
Balar, A.V., Weber, J.S. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 66, 551–564 (2017). https://doi.org/10.1007/s00262-017-1954-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1954-6