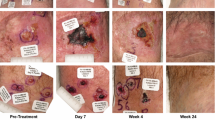
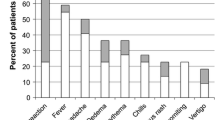
Abstract
Intralesional therapy of melanoma patients with locally advanced metastatic disease is attracting increasing interest, not least due to its ability to lead to both direct tumor cell killing and the stimulation of both a local and a systemic immune response. An obvious pre-requisite for this type of approach is the presence of accessible metastases that are amenable to direct injection with the therapeutic agent of interest. Patients who present with these characteristics belong to stages IIIB/C or IV of the disease. Surgical resection with intention to cure is the standard of care for patients with limited tumor burden and confined spread of disease (resectable patients). However, this category of patients is at a high risk of further recurrences until the disease becomes inoperable (unresectable) or progresses to a more advanced stage with visceral organ involvement, after which the prognosis is particularly grim. Most of the intralesional treatments tested so far, including the recently approved oncolytic virus talimogene laherparepvec, target the subpopulation of patients with unresectable disease, but the possibility to use the intralesional treatment in a neoadjuvant setting for fully resectable patients is attracting considerable interest. The present article reviews approved products and advanced stage pharmaceutical agents in development for the intralesional treatment of melanoma patients.


Similar content being viewed by others
Abbreviations
- AEs:
-
Adverse events
- CR:
-
Complete responses
- CTLA-4:
-
Cytotoxic T lymphocyte antigen 4
- DLT:
-
Dose-limiting toxicity
- DPCP:
-
Diphencyprone
- ECOG:
-
Eastern Cooperative Oncology Group
- EMA:
-
European medicine agency
- FDA:
-
Food and drug administration
- IFNa:
-
Interferon α
- IFNb:
-
Interferon β
- IL2:
-
Interleukin-2
- ILP:
-
Isolated limb perfusion
- irRC:
-
Immune-related response criteria
- ITT:
-
Intention-to-treat
- OR:
-
Objective response
- ORR:
-
Objective response rate
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PV-10:
-
Rose Bengal (Provectus)
- RFS:
-
Recurrence-free survival
- TLR7:
-
Toll-like receptor 7
- TNF:
-
Tumor necrosis factor α
- T-Vec:
-
Talimogene laherparepvec, Imlygic™ (Amgen)
References
Geller AC, Annas GD (2003) Epidemiology of melanoma and nonmelanoma skin cancer. Semin Oncol Nurs 19(1):2–11
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403. doi:10.1016/j.ejca.2012.12.027
Balch CM, Gershenwald JE, Soong SJ et al (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27(36):6199–6206. doi:10.1200/JCO.2009.23.4799
Essner R, Lee JH, Wanek LA, Itakura H, Morton DL (2004) Contemporary surgical treatment of advanced-stage melanoma. Arch Surg 139(9):961–966. doi:10.1001/archsurg.139.9.961 (discussion 966–967)
Lee CC, Faries MB, Wanek LA, Morton DL (2009) Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol 27(21):3489–3495. doi:10.1200/JCO.2008.18.9845
Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, Garbe C (2002) Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol 147(1):62–70
Abbott AM, Zager JS (2014) Locoregional therapies in melanoma. Surg Clin North Am 94(5):1003–1015, viii. doi:10.1016/j.suc.2014.07.004
Andtbacka RH, Kaufman HL, Collichio F et al (2015) Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33(25):2780–2788. doi:10.1200/JCO.2014.58.3377
Weide B, Derhovanessian E, Pflugfelder A, Eigentler TK, Radny P, Zelba H, Pfohler C, Pawelec G, Garbe C (2010) High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer 116(17):4139–4146. doi:10.1002/cncr.25156
Mastrangelo MJ, Bellet RE, Berkelhammer J, Clark WH Jr (1975) Regression of pulmonary metastatic disease associated with intralesional BCG therapy of intracutaneous melanoma metastases. Cancer 36(4):1305–1308
Boyd KU, Wehrli BM, Temple CL (2011) Intra-lesional interleukin-2 for the treatment of in-transit melanoma. J Surg Oncol 104(7):711–717. doi:10.1002/jso.21968
Dehesa LA, Vilar-Alejo J, Valeron-Almazan P, Carretero G (2009) [Experience in the treatment of cutaneous in-transit melanoma metastases and satellitosis with intralesional interleukin-2]. Actas Dermosifiliogr 100(7):571–585
Gutwald JG, Groth W, Mahrle G (1994) Peritumoral injections of interleukin 2 induce tumour regression in metastatic malignant melanoma. Br J Dermatol 130(4):541–542
Radny P, Caroli UM, Bauer J, Paul T, Schlegel C, Eigentler TK, Weide B, Schwarz M, Garbe C (2003) Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer 89(9):1620–1626. doi:10.1038/sj.bjc.6601320
Weide B, Eigentler TK, Pflugfelder A et al (2014) Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res 2(7):668–678. doi:10.1158/2326-6066.CIR-13-0206
Pretto F, Elia G, Castioni N, Neri D (2014) Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunol Immunother 63(9):901–910. doi:10.1007/s00262-014-1562-7
Marabelle A, Kohrt H, Caux C, Levy R (2014) Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res 20(7):1747–1756. doi:10.1158/1078-0432.CCR-13-2116
Temizoz B, Kuroda E, Ishii KJ (2016) Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol 28(7):329–338. doi:10.1093/intimm/dxw015
Kaufman HL, Kohlhapp FJ, Zloza A (2015) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14(9):642–662. doi:10.1038/nrd4663
Karakousis CP, Douglass HO Jr, Yeracaris PM, Holyoke ED (1976) BCG immunotherapy in patients with malignant melanoma. Arch Surg 111(6):716–718
Robinson JC (1977) Risks of BCG intralesional therapy: an experience with melanoma. J Surg Oncol 9(6):587–593
Barth A, Morton DL (1995) The role of adjuvant therapy in melanoma management. Cancer 75(2 Suppl):726–734
Seigler HF, Shingleton WW, Pickrell KL (1975) Intralesional BCG, intravenous immune lymphocytes, and immunization with neuraminidase-treated tumor cells to manage melanoma; a clinical assessment. Plast Reconstr Surg 55(3):294–298
Agarwala SS, Neuberg D, Park Y, Kirkwood JM (2004) Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer 100(8):1692–1698. doi:10.1002/cncr.20166
Damian DL, Shannon KF, Saw RP, Thompson JF (2009) Topical diphencyprone immunotherapy for cutaneous metastatic melanoma. Australas J Dermatol 50(4):266–271. doi:10.1111/j.1440-0960.2009.00556.x
Kidner TB, Morton DL, Lee DJ, Hoban M, Foshag LJ, Turner RR, Faries MB (2012) Combined intralesional Bacille Calmette-Guerin (BCG) and topical imiquimod for in-transit melanoma. J Immunother 35(9):716–720. doi:10.1097/CJI.0b013e31827457bd
Hemmi H, Kaisho T, Takeuchi O et al (2002) Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3(2):196–200. doi:10.1038/ni758
Walter A, Schafer M, Cecconi V et al (2013) Aldara activates TLR7-independent immune defence. Nat Commun 4:1560. doi:10.1038/ncomms2566
Miller RL, Gerster JF, Owens ML, Slade HB, Tomai MA (1999) Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol 21(1):1–14
Bilu D, Sauder DN (2003) Imiquimod: modes of action. Br J Dermatol 149(Suppl 66):5–8
Sisti A, Sisti G, Oranges CM (2015) Topical treatment of melanoma skin metastases with Imiquimod: a review. Dermatol Online J 21(2)
Florin V, Desmedt E, Vercambre-Darras S, Mortier L (2012) Topical treatment of cutaneous metastases of malignant melanoma using combined imiquimod and 5-fluorouracil. Invest New Drugs 30(4):1641–1645. doi:10.1007/s10637-011-9717-2
Buckley DA, Du Vivier AW (2001) The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol 145(3):385–405
van der Steen PH, Happle R (1993) Topical immunotherapy of alopecia areata. Dermatol Clin 11(3):619–622
Damian DL, Saw RP, Thompson JF (2014) Topical immunotherapy with diphencyprone for in transit and cutaneously metastatic melanoma. J Surg Oncol 109(4):308–313. doi:10.1002/jso.23506
He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B (1997) Suppression of the phenotype of gamma(1)34.5-herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J Virol 71(8):6049–6054
Liu BL, Robinson M, Han ZQ et al (2003) ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 10(4):292–303. doi:10.1038/sj.gt.3301885
Hu JC, Coffin RS, Davis CJ et al (2006) A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 12(22):6737–6747. doi:10.1158/1078-0432.CCR-06-0759
Senzer NN, Kaufman HL, Amatruda T et al (2009) Phase II clinical trial of a granulocyte–macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27(34):5763–5771. doi:10.1200/JCO.2009.24.3675
Chen L, Daud A (2016) A review of novel intralesional therapies for melanoma, with an emphasis on a potential combination approach. Oncology (Williston Park) 30(5):442–443
Agarwala SS (2015) Intralesional therapy for advanced melanoma: promise and limitation. Curr Opin Oncol 27(2):151–156. doi:10.1097/CCO.0000000000000158
Engeland CE, Grossardt C, Veinalde R et al (2014) CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther 22(11):1949–1959. doi:10.1038/mt.2014.160
Puzanov I, Milhem MM, Minor D et al (2016) Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIb-IV melanoma. J Clin Oncol 34(22):2619–2626. doi:10.1200/JCO.2016.67.1529
Long GV, Dummer R, Ribas A et al (2016) Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol 34, (suppl; abstr TPS9598)
Mousavi H, Zhang X, Gillespie S, Wachter E, Hersey P (2006) Rose Bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. Melanoma Res 16:S8
Thompson JF, Hersey P, Wachter E (2008) Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res 18(6):405–411. doi:10.1097/CMR.0b013e32831328c7
Wiener M, Damian DL, Thompson JF (2008) Systemic phototoxicity following intralesional rose bengal for subcutaneous melanoma metastases. Dermatology 216(4):361–362. doi:10.1159/000117707
Thompson JF, Agarwala SS, Smithers BM et al (2015) Phase 2 study of intralesional pv-10 in refractory metastatic melanoma. Ann Surg Oncol 22(7):2135–2142. doi:10.1245/s10434-014-4169-5
Schrama D, Reisfeld RA, Becker JC (2006) Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov 5(2):147–159. doi:10.1038/nrd1957
Johannsen M, Spitaleri G, Curigliano G et al (2010) The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer 46(16):2926–2935. doi:10.1016/j.ejca.2010.07.033
Eigentler TK, Weide B, de Braud F et al (2011) A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res 17(24):7732–7742. doi:10.1158/1078-0432.CCR-11-1203
Spitaleri G, Berardi R, Pierantoni C et al (2013) Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol 139(3):447–455. doi:10.1007/s00432-012-1327-7
Papadia F, Basso V, Patuzzo R et al (2013) Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol 107(2):173–179. doi:10.1002/jso.23168
Schwager K, Hemmerle T, Aebischer D, Neri D (2013) The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol 133(3):751–758. doi:10.1038/jid.2012.376
Danielli R, Patuzzo R, Di Giacomo AM et al (2015) Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother 64(8):999–1009. doi:10.1007/s00262-015-1704-6
Johnson DB, Sosman JA (2015) Therapeutic advances and treatment options in metastatic melanoma. JAMA Oncol 1(3):380–386. doi:10.1001/jamaoncol.2015.0565
Hauschild A, Gogas H, Tarhini A, Middleton MR, Testori A, Dreno B, Kirkwood JM (2008) Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer 112(5):982–994. doi:10.1002/cncr.23251
Lotze MT (1995) Biologic therapy with interleukin-2: Preclinical studies. In: DeVita VTJ, Hellman S, Rosenberg SA (eds) Biologic therapy of cancer. Lippincott, Philadelphia, pp 207–233
Rosenberg SA, Lotze MT, Yang JC et al (1989) Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol 7(12):1863–1874
van Horssen R, Ten Hagen TL, Eggermont AM (2006) TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 11(4):397–408. doi:10.1634/theoncologist.11-4-397
Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM (2015) Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33(7):773–781. doi:10.1200/JCO.2014.57.4756
Acknowledgements
Support from the Swiss Federal Institute of Technology Zurich (ETHZ), the Swiss National Science Foundation (SNF) and from European Research Council (ERC) Advanced Grant (ZAUBERKUGEL) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Weide declares receiving commercial research grants from Bristol-Myers Squibb (BMS) and Merck Sharp and Dohme (MSD), having received travel/accommodation expenses from BMS, MSD, Roche, Amgen, Philogen, Curevac and compensation for advisory services from MSD, BMS, Philogen, and Curevac. D. Neri is co-founder and shareholder of Philogen S.p.A. G. Elia declares no conflict of interest.
Additional information
This paper is a Focussed Research Review based on a presentation given at the Fourteenth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 10th–12th May, 2016. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
Rights and permissions
About this article
Cite this article
Weide, B., Neri, D. & Elia, G. Intralesional treatment of metastatic melanoma: a review of therapeutic options. Cancer Immunol Immunother 66, 647–656 (2017). https://doi.org/10.1007/s00262-016-1952-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1952-0