Abstract
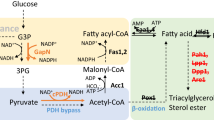
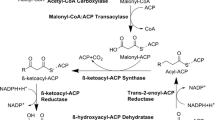
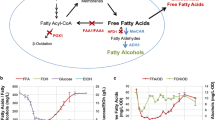
Production of biofuels derived from microbial fatty acids has attracted great attention in recent years owing to their potential to replace petroleum-derived fuels. To be cost competitive with current petroleum fuel, flux toward the direct precursor fatty acids needs to be enhanced to approach high yields. Herein, fatty acyl-CoA metabolism in Saccharomyces cerevisiae was engineered to accumulate more free fatty acids (FFA). For this purpose, firstly, haploid S. cerevisiae double deletion strain △faa1△faa4 was constructed, in which the genes FAA1 and FAA4 encoding two acyl-CoA synthetases were deleted. Then the truncated version of acyl-CoA thioesterase ACOT5 (Acot5s) encoding Mus musculus peroxisomal acyl-CoA thioesterase 5 was expressed in the cytoplasm of the strain △faa1△faa4. The resulting strain △faa1△faa4 [Acot5s] accumulated more extracellular FFA with higher unsaturated fatty acid (UFA) ratio as compared to the wild-type strain and double deletion strain △faa1△faa4. The extracellular total fatty acids (TFA) in the strain △faa1△faa4 [Acot5s] increased to 6.43-fold as compared to the wild-type strain during the stationary phase. UFA accounted for 42 % of TFA in the strain △faa1△faa4 [Acot5s], while no UFA was detected in the wild-type strain. In addition, the expression of Acot5s in △faa1△faa4 restored the growth, which indicates that FFA may not be the reason for growth inhibition in the strain △faa1△faa4. RT-PCR results demonstrated that the de-repression of fatty acid synthesis genes led to the increase of extracellular fatty acids. The study presented here showed that through control of the acyl-CoA metabolism by deleting acyl-CoA synthetase and expressing thioesterase, more FFA could be produced in S. cerevisiae, demonstrating great potential for exploitation in the platform of microbial fatty acid-derived biofuels.







Similar content being viewed by others
References
Black PN, DiRusso CC (2007) Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta Mol Cell Biol Lipids 1771(3):286–298. doi:10.1016/j.bbalip.2006.05.003
Bortz WM, Lynen F (1963) The inhibition of acetyl CoA carboxylase by long chain acyl CoA derivatives. Biochem Z 337:505–509
Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152(1):141–145. doi:10.1016/0003-2697(86)90132-6
Chen L, Zhang J, Chen WN (2014) Engineering the Saccharomyces cerevisiae β-oxidation pathway to increase medium chain fatty acid production as potential biofuel. PLoS ONE 9(1):e84853. doi:10.1371/journal.pone.0084853
Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, Fisk DG, Hirschman JE, Hitz BC, Karra K, Krieger CJ, Miyasato SR, Nash RS, Park J, Skrzypek MS, Simison M, Weng S, Wong ED (2012) Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res 40(D1):D700–D705. doi:10.1093/nar/gkr1029
Choi YJ, Lee SY (2013) Microbial production of short-chain alkanes. Nature 502(7472):571–574. doi:10.1038/nature12536 http://www.nature.com/nature/journal/v502/n7472/abs/nature12536.html#supplementary-information
Choi J-Y, Stukey J, Hwang S-Y, Martin CE (1996) Regulatory elements that control transcription activation and unsaturated fatty acid-mediated repression of the Saccharomyces cerevisiae OLE1 gene. J Biol Chem 271(7):3581–3589. doi:10.1074/jbc.271.7.3581
Faergeman NJ, Knudsen J (1997) Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J 323:1–12
Færgeman NJ, Black PN, Zhao XD, Knudsen J, DiRusso CC (2001) The acyl-CoA synthetases encoded within FAA1 and FAA4 in Saccharomyces cerevisiae function as components of the fatty acid transport system linking import, activation, and intracellular utilization. J Biol Chem 276(40):37051–37059. doi:10.1074/jbc.M100884200
Feddersen S, Neergaard TBF, Knudsen J, Faergeman NJ (2007) Transcriptional regulation of phospholipid biosynthesis is linked to fatty acid metabolism by an acyl-CoA-binding-protein-dependent mechanism in Saccharomyces cerevisiae. Biochem J 407:219–230. doi:10.1042/bj20070315
Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH (2002) A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30(6):e23
Hamilton J, Guo W, Kamp F (2002) Mechanism of cellular uptake of long-chain fatty acids: do we need cellular proteins? In: Glatz JC (ed) Cellular lipid binding proteins. Dev Mol Cell Biochem vol 38. Springer US, pp 17–23
Horak T, Culik J, Cejka P, Jurkova M, Kellner V, Dvorak J, Haskova D (2009) Analysis of free fatty acids in beer: comparison of solid-phase extraction, solid-phase microextraction, and stir bar sorptive extraction. J Agric Food Chem 57(23):11081–11085. doi:10.1021/jf9028305
Howard TP, Middelhaufe S, Moore K, Edner C, Kolak DM, Taylor GN, Parker DA, Lee R, Smirnoff N, Aves SJ, Love J (2013) Synthesis of customized petroleum-replica fuel molecules by targeted modification of free fatty acid pools in Escherichia coli. Proc Natl Acad Sci. doi:10.1073/pnas.1215966110
Jiang P, Cronan JE (1994) Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacteriol 176(10):2814–2821
Jones JM, Nau K, Geraghty MT, Erdmann R, Gould SJ (1999) Identification of peroxisomal acyl-CoA thioesterases in yeast and humans. J Biol Chem 274(14):9216–9223. doi:10.1074/jbc.274.14.9216
Kamiryo T, Parthasarathy S, Numa S (1976) Evidence that acyl coenzyme A synthetase activity is required for repression of yeast acetyl coenzyme A carboxylase by exogenous fatty acids. Proc Natl Acad Sci U S A 73(2):386–390
Kamisaka Y, Kimura K, Uemura H, Yamaoka M (2013) Overexpression of the active diacylglycerol acyltransferase variant transforms Saccharomyces cerevisiae into an oleaginous yeast. Appl Microbiol Biotechnol 97(16):7345–7355. doi:10.1007/s00253-013-4915-9
Kamp F, Hamilton JA (2006) How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fat Acids 75(3):149–159. doi:10.1016/j.plefa.2006.05.003
Knoll LJ, Johnson DR, Gordon JI (1994) Biochemical studies of three Saccharomyces cerevisiae acyl-CoA synthetases, Faa1p, Faa2p, and Faa3p. J Biol Chem 269(23):16348–16356
Knoll LJ, Johnson DR, Gordon JI (1995) Complementation of Saccharomyces cerevisiae strains containing fatty acid activation gene (FAA) deletions with a mammalian acyl-CoA synthetase. J Biol Chem 270(18):10861–10867. doi:10.1074/jbc.270.18.10861
Krivoruchko A, Siewers V, Nielsen J (2011) Opportunities for yeast metabolic engineering: lessons from synthetic biology. Biotechnol J 6(3):262–276. doi:10.1002/biot.201000308
Leber C, Da Silva NA (2013) Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnol Bioeng. doi:10.1002/bit.25021
Leibundgut M, Maier T, Jenni S, Ban N (2008) The multienzyme architecture of eukaryotic fatty acid synthases. Curr Opin Struct Biol 18(6):714–725. doi:10.1016/j.sbi.2008.09.008
Li H, Melton EM, Quackenbush S, DiRusso CC, Black PN (2007) Mechanistic studies of the long chain acyl-CoA synthetase Faa1p from Saccharomyces cerevisiae. Biochim Biophys Acta Mol Cell Biol Lipids 1771(9):1246–1253. doi:10.1016/j.bbalip.2007.05.009
Liu J-F, Nie K-L, Fan L-H, Wang F, Tan T-W, Deng L (2013) Increased production of FAEEs for biodiesel with lipase enhanced Saccharomyces cerevisiae. Process Biochem 48(8):1212–1215. doi:10.1016/j.procbio.2013.06.003
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Lu XF, Vora H, Khosla C (2008) Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab Eng 10(6):333–339. doi:10.1016/j.ymben.2008.08.006
Maeda I, Delessert S, Hasegawa S, Seto Y, Zuber S, Poirier Y (2006) The peroxisomal acyl-CoA thioesterase Pte1p from Saccharomyces cerevisiae is required for efficient degradation of short straight chain and branched chain fatty acids. J Biol Chem 281(17):11729–11735. doi:10.1074/jbc.M511762200
Magnuson K, Jackowski S, Rock CO, Cronan JE (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 57(3):522–542
Martin CE, Oh C-S, Jiang Y (2007) Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochim Biophys Acta Mol Cell Biol Lipids 1771(3):271–285
Oshiro T, Aiba H, Mizuno T (2003) A defect in a fatty acyl-CoA synthetase gene, lcf1+, results in a decrease in viability after entry into the stationary phase in fission yeast. Mol Gen Genomics 269(4):437–442. doi:10.1007/s00438-003-0841-3
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100(1):261–268. doi:10.1016/j.biortech.2008.06.039
Ramsay RR, Gandour RD, van der Leij FR (2001) Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta Protein Struct Mol Enzymol 1546(1):21–43
Russell Johnson D, Knoll LJ, Levin DE, Gordon JI (1994) Saccharomyces cerevisiae contains four fatty acid activation (FAA) genes: an assessment of their role in regulating protein N-myristoylation and cellular lipid metabolism. J Cell Biol 127(3):751–762
Scharnewski M, Pongdontri P, Mora G, Hoppert M, Fulda M (2008) Mutants of Saccharomyces cerevisiae deficient in acyl-CoA synthetases secrete fatty acids due to interrupted fatty acid recycling. FEBS J 275(11):2765–2778. doi:10.1111/j.1742-4658.2008.06417.x
Schirmer A, Rude MA, Li XZ, Popova E, del Cardayre SB (2010) Microbial biosynthesis of alkanes. Science 329(5991):559–562. doi:10.1126/science.1187936
Sorger D, Daum G (2002) Synthesis of triacylglycerols by the acyl-coenzyme A: diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J Bacteriol 184(2):519–524. doi:10.1128/jb.184.2.519-524.2002
Steen EJ, Kang YS, Bokinsky G, Hu ZH, Schirmer A, McClure A, del Cardayre SB, Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463(7280):559–562. doi:10.1038/nature08721
Sumper M, Träuble H (1973) Membranes as acceptors for palmitoyl CoA in fatty acid biosynthesis. FEBS Lett 30(1):29–34. doi:10.1016/0014-5793(73)80612-X
Tang X, Feng H, Chen WN (2013) Metabolic engineering for enhanced fatty acids synthesis in Saccharomyces cerevisiae. Metab Eng 16:95–102. doi:10.1016/j.ymben.2013.01.003
Torella JP, Ford TJ, Kim SN, Chen AM, Way JC, Silver PA (2013) Tailored fatty acid synthesis via dynamic control of fatty acid elongation. Proc Natl Acad Sci 110(28):11290–11295. doi:10.1073/pnas.1307129110
Voelker TA, Davies HM (1994) Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J Bacteriol 176(23):7320–7327
Wang MX, Bai J, Chen WN, Ching CB (2010) Metabolomic profiling of cellular responses to carvedilol enantiomers in vascular smooth muscle cells. PLoS ONE 5(11):e15441. doi:10.1371/journal.pone.0015441
Westin MAK, Alexson SEH, Hunt MC (2004) Molecular cloning and characterization of two mouse peroxisome proliferator-activated receptor α (PPARα)-regulated peroxisomal acyl-CoA thioesterases. J Biol Chem 279(21):21841–21848. doi:10.1074/jbc.M313863200
Zheng Y-N, Li L-L, Liu Q, Yang J-M, Wang X-W, Liu W, Xu X, Liu H, Zhao G, Xian M (2012) Optimization of fatty alcohol biosynthesis pathway for selectively enhanced production of C12/14 and C16/18 fatty alcohols in engineered Escherichia coli. Microb Cell Factories 11(1):65
Acknowledgments
We thank Prof. J. H. Hegemann for kindly providing the plasmids for gene deletion. This research is supported by a Competitive Research Programme (CRP) grant from the National Research Foundation of Singapore.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 96 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Zhang, J., Lee, J. et al. Enhancement of free fatty acid production in Saccharomyces cerevisiae by control of fatty acyl-CoA metabolism. Appl Microbiol Biotechnol 98, 6739–6750 (2014). https://doi.org/10.1007/s00253-014-5758-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5758-8