Conclusions
-
1.
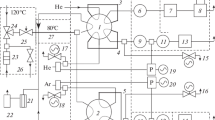
The completeness of the oxidation of an organic substance by oxides in a flow-type system depends not only upon the effectiveness of the oxidizing agent, but also upon the rate of delivery of the substance to the oxidizing layer and upon the physical and chemical properties of the substance. In the classical method of decomposition of the sample, identical conditions guaranteeing an equal degree of oxidation of various substances cannot be ensured.
-
2.
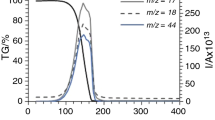
In decomposition in a semiclosed system (with delivery of the products of thermal decomposition of the substance to the oxygen-rich zone), complete oxidation of the organic substance is ensured independent of the use of an oxidizing agent.
-
3.
When the oxidation was conducted in an inert gas atmosphere, nickel oxide proved to be the most active of the tested oxidizing agents.
Similar content being viewed by others
Literature cited
J. Horacek, J. Korbl, and V. Pechanec, Collect. Czechoslov. Chem. Commun.,27, 1254 (1962).
M. Vecera, D. Xnobl, and L. Synek, Mikrochim. Acta, 370 (1961).
G. Kainz and L. Hainberger, Z. Analyt. Chem.,169, 406 (1959).
A. A. Duswalt and W. W. Brant, Analyt. Chem.,32, 272 (1960).
L. T. Marvin and M. G. Grant, Microchem. J., 175 (1966).
W. Walish, Trans. N. Y. Acad. Sci.,25, No. 7, 693 (1963).
Technicon Controls Incorporated. Chem. Process (Engl.),9, No. 8, 28 (1963).
H. Weitkamp and F. Korte, Chem.-Ing. Techn.,35, 429 (1963).
J. T. Clerc and W. Simon, Mikrochem. J.,7, 422 (1963).
K. Hozumi, Microchem. J.,46 (1966).
J. Howard, Jr., Francis, Analyt. Chem.,36, No. 7, 31A (1964).
A. Kurtenacker, Z. Analyt. Chem.,50, 548 (1911).
W. J. Kirsten, Analyt. Chem.,19, 925 (1947).
W. J. Kirsten, Mikrochim. Acta, 840 (1956).
M. O. Korshun and V. A. Klimova, Zh. Analiticheskoi Khim.,2, 274 (1947).
R. Belcher and C. E. Spooner, Fuel,20, 130 (1941).
L. I. Lebedeva and E. F. Fedorova, Zh. Analiticheskoi Khim.,16, 87 (1961).
E. Ruf, Z. Analyt. Chem.,163, 21 (1958).
M. Vecera and L. Sinek, Collect. Czechoslov. Chem. Commun.,24, 3402 (1959).
M. Vecera and L. Sinek, Mikrochim. Acta, 208 (1960).
M. Vecera, Mikrochim. Acta, 196 (1964).
W. J. Kirsten, Mikrochem.,40, 121 (1953).
M. N. Chumachenko, Izv. Akad. Nauk SSSR, Ser. Khim., 1893 (1963).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 2, pp. 235–239, February, 1968.
Rights and permissions
About this article
Cite this article
Chumachenko, M.N., Pakhomova, I.E. Method of decomposition of an organic substance with simultaneous determination of carbon, hydrogen, and nitrogen by the gas chromatography method. Russ Chem Bull 17, 235–238 (1968). https://doi.org/10.1007/BF00908417
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00908417