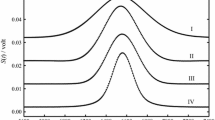
Abstract
Measurements of mutual diffusion coefficients at infinite dilution in aqueous solutions of alkali-metal chlorides have been made with the Taylor dispersion technique. Data were obtained for the series LiCl/H2O, NaCl/H2O, KCl/H2O, RbCl/H2O, and CsCl/H2O, at five temperatures between 298.15 and 318.15 K. A linear dependence with temperature was found. This technique is very convenient in comparison with other more time-consuming techniques.
Similar content being viewed by others
References
H. J. V. Tyrrell and K. R. Harris, Diffusion in Liquids (Butterworths, London, 1984).
J. W. McBain and T. H. Liu, J. Am. Chem. Soc. 53:59 (1931).
R. H. Stokes, J. Am. Chem. Soc. 72:763 (1950).
R. H. Stokes, J. Am. Chem. Soc. 72:2243 (1950).
R. H. Stokes, J. Am. Chem. Soc. 73:3527 (1951).
T. Hashitani and R. Tamamushi, Trans. Faraday Soc. 63:369 (1967).
K. Tanaka, T. Hashitani, and R. Tamamushi, Trans. Faraday. Soc. 66:74 (1970).
S. K. Jalota and R. Paterson, J. Chem. Soc. Faraday Trans. 69:1510 (1973).
J. H. Northrop and M. L. Anson, J. Gen. Physiol. 12:543 (1929).
H. S. Harned and D. M. French, Ann. N.Y. Acad. Sci. 44:267 (1945).
H. S. Harned and R. L. Nutall, J. Am. Chem. Soc. 69:736 (1947).
H. S. Harned and R. L. Nutall, J. Am. Chem. Soc. 71:1460 (1949).
H. S. Harned and C. L. Hildreth, J. Am. Chem. Soc. 73:650 (1951).
K. Kamakura, Bull. Chem. Soc. Jpn. 55:3353 (1982).
J. N. Agar and V. M. Lobo, J. Chem. Soc. Faraday I 71:1659 (1975).
C. Durou, C. Moutou, and J. Mahenc, J. Chim. Phys. 71:271 (1971).
J. G. Becsey and J. A. Bierlein, Rev. Sci. Instrum. 49:227 (1978).
J. A. Rard and D. G. Miller, J. Chem. Eng. Data 25:211 (1980).
J. A. Rard and D. G. Miller, J. Chem. Soc. Faraday Trans. 78:887 (1982).
H. S. Dunsmore, S. K. Jalota, and R. Paterson, J. Chem. Soc. A 1061 (1969).
J. H. Wang and J. W. Kennedy, J. Am. Chem. Soc. 72:2080 (1950).
E. Kumamoto and H. Kimizuka, Bull. Chem. Soc. Jpn. 52:2145 (1979).
D. G. Miller, J. Phys. Chem. 70:2639 (1966).
G. I. Taylor, Proc. Roy. Soc. A 219:186 (1953).
A. Alizadeh, C. A. Nieto de Castro, and W. A. Wakeham, Int. J. Thermophys. 1:243 (1980).
A. Alizadeh and W. A. Wakeham, Int. J. Thermophys. 3:307 (1982).
C. M. Padrel de Oliveira, J. M. N. A. Fareleira, and C. A. Nieto de Castro, Int. J. Thermophys. 10:973 (1989).
M. L. S. Matos Lopez, C. A. Nieto de Castro, and J. V. Sengers, Int. J. Thermophys. 13:283 (1992).
R. Castillo, H. Dominguez, and M. Costas, J. Phys. Chem. 94:8731 (1990).
R. Castillo, C. Garza, and J. Orozco, J. Phys. Chem. 96:1476 (1992).
R. A. Robinson and R. H. Stokes, Electrolyte Solutions, 2nd ed. (Butterworths, London, 1970).
J. Koryta and J. Dvorak, Principles of Electrochemistry (Wiley, New York, 1987).
S. I. Smedley, The Interpretation of Ionic Conductivity in Liquids (Penum, New York, 1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Castillo, R., Garza, C. Temperature dependence of the mutual diffusion coefficients in aqueous solutions of alkali metal chlorides. Int J Thermophys 14, 1145–1152 (1993). https://doi.org/10.1007/BF00503678
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00503678